World health organization presentation
World health organization presentation
World Health Organization
Published byAshtyn Mullins Modified over 7 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
7 April, 2017 International Health Regulations Building international public health security
2 Why revised International Health Regulations?
World Health Organization Why revised International Health Regulations? 7 April, 2017 In today’s world, diseases travel fast and no single country can protect itself on its own. Acknowledging this, the 193 WHO Member States unanimously adopted a new version of the International Health Regulations (IHR). The revised IHR enter into force in June It will now be up to the world to translate the new code of the Regulations into the reality of greater international public health security. Dr Margaret Chan, WHO Director-General
3 Our world is changing as never before
World Health Organization 7 April, 2017 Populations grow, age, and move Diseases travel fast Microbes adapt Chemical, radiation, food risks increase Health security is at stake The unique conditions of the 21st century have amplified the invasive and disruptive power of epidemics and other public health emergencies. The dynamics of disease spread in the world have changed greatly in the last decades. We all are living in a global “village” where diseases can travel at the speed of jetliners on the wings of international travel and trade, and can jump from one continent to another in a matter of hours. This has made all nations vulnerable – not just to invasion of their territories by pathogens, but also to the economic, political and social shocks of public health events elsewhere. They have the power to disrupt the entire global system in ways that cannot be controlled by one nation acting alone. SARS was the first disease of the 21st century to expose the world’s vulnerabilities. It will not be the last. Shared vulnerabilities imply shared responsibilities and create a need for strong collective action to protect lives and livelihoods from disease spread. To address these public health risks, the world’s countries, through WHO, initiated an intensive process to revise the IHR, eventually adopted by the World Health Assembly in May 2005.
5 2003: SARS changes the world
World Health Organization 7 April, 2017 Screening of exit passengers WHO travel recommendations WHO travel recommendations removed 27 March 2 April 25 May 23 June 120000 SARS: an unknown coronavirus 8098 cases 774 deaths 26 countries affected trends in airline passenger movement drop economic loss: US$ 60 billion 100000 80000 Number of passenger 60000 SARS taught us how quickly a new disease can spread along the routes of international air travel. This universal vulnerability creates a need for collective defences and for shared responsibility in making these defences work. SARS finally spurred the energy of world’s States, coordinated by WHO, to strongly take action and strengthen global defences to health threats without boundaries 40000 36 116 20000 14 670 13 May 3/16 3/19 3/22 3/25 3/28 3/31 4/3 4/6 4/9 4/12 4/15 4/18 4/21 4/24 4/27 4/30 5/3 5/6 5/9 5/12 5/15 5/18 5/21 5/24 5/27 5/30 6/2 6/5 6/8 6/11 6/14 6/17
6 H5N1: Avian influenza, a pandemic threat
World Health Organization 7 April, 2017 The greatest threat to international health security would be an influenza pandemic. It has not receded, but early warnings allow the world a chance to prepare. Implementation of the IHR is the chance to prepare
7 World Health Organization
The 58th World Health Assembly adopts the revised International Health Regulations, “IHR” World Health Organization 7 April, 2017
9 World Health Organization
What’s new? World Health Organization 7 April, 2017 From three diseases to all public health threats From preset measures to adapted response From control of borders to, also, containment at source The IHR are innovative because they move from purely a list of diseases to a dynamic process of risk identification, assessment and management they move from a concept of static defence at borders, airports and ports to the concept of early detection, reporting and containment at source they built on the concept that international health security is based on strong national public health infrastructure connected a global alert and response system.
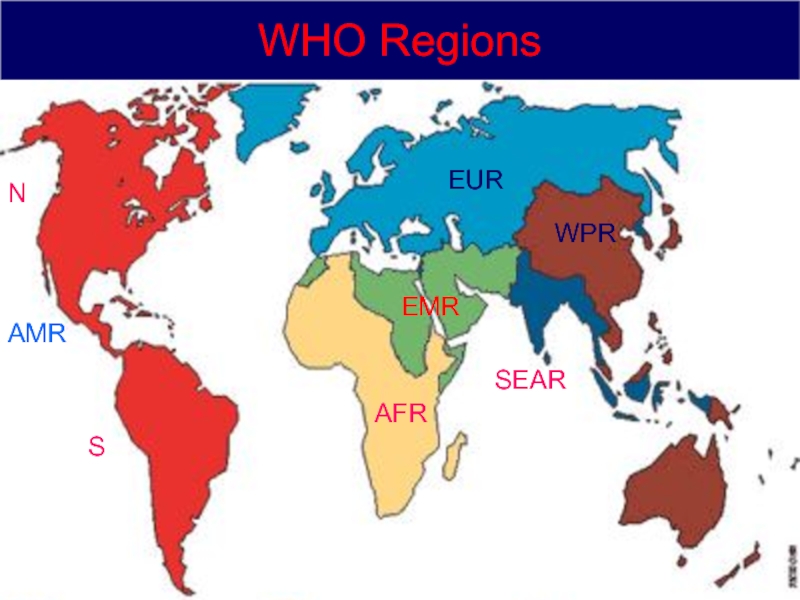
10 All public health threats
World Health Organization All public health threats 7 April, 2017 The revised IHR recognize that international disease threats have increased Scope has been expanded from cholera, plague and yellow fever to all public health emergencies of international concern They include those caused by infectious diseases, chemical agents, radioactive materials and contaminated food
11 World Health Organization
Adapted response World Health Organization 7 April, 2017 International public health security is based on strong national public health infrastructure connected to a global alert and response system. This is at the core of the IHR. SARS taught us how quickly a new disease can spread along the routes of international air travel. This universal vulnerability creates a need for collective defences and for shared responsibility in making these defences work. The containment of SARS was due to unprecedented cooperation between countries which prevented a new disease gaining a foothold in the human population. The IHR build on this and many other efforts to respond to and contain disease threats and will help to ensure that outbreaks and other public health emergencies are detected and investigated more rapidly and that collective international action is taken to support affected a states to contain the disease and apply life saving interventions. GOARN A “strike force” of specialized staff is rapidly deployed for on-the-spot emergency investigations through the Global Outbreak Alert and Response Network (GOARN). It interlinks in real time 120 networks and institutes. For chemical, radiological and food-related incidents GOARN is complemented by WHO Chemical Incidents and Emergencies Network (ChemiNet) Radiation Emergency Medical Preparedness and Assistance Network (REMPAN) International Food Safety Authorities Network (INFOSAN)
12 World Health Organization
Containment at source World Health Organization 7 April, 2017 Rapid response at the source is: the most effective way to secure maximum protection against international spread of diseases key to limiting unnecessary health-based restrictions on trade and travel The extent of international travels is an extraordinary opportunity for disease transmission.
13 World Health Organization
What do the IHR call for? 7 April, 2017 Strengthened national capacity for surveillance and control, including in travel and transport Prevention, alert and response to international public health emergencies Global partnership and international collaboration Rights, obligations and procedures, and progress monitoring
14 Why should countries implement the IHR?
World Health Organization Why should countries implement the IHR? 7 April, 2017 To detect and contain public health threats faster, to contribute to international public health security, and to enjoy the benefits of being a respected partner. Countries will receive: WHO assistance in building core capacities WHO’s guidance during outbreak investigation, risk assessment, and response WHO’s advice and logistical support information gathered by WHO about public health risks worldwide assistance to mobilize funding support
15 The IHR foster global partnership
World Health Organization 7 April, 2017 Other intergovernmental organizations: UN system (e.g. FAO, IAEA, ICAO, IMO) others: regional (e.g. EU, ASEAN), technical (e.g. OIE) Development agencies: governments, banks WHO Collaborating centres Academics & professional associations Industry associations NGOs and Foundations The IHR provide for cooperation between WHO and other competent intergovernmental organizations or international bodies in the implementation of the Regulations. WHO will therefore continue to foster its longstanding working relationships with a number of organizations such as the International Atomic Energy Agency, the International Air Transport Association, the International Civil Aviation Organization, the International Maritime Organization, the World Tourism Organization, Food and Agriculture Organization of the United Nations, the Office International d’Epizooties (World Organisation for Animal Health) and the World Trade Organization. In addition to these specialized organizations, WHO will work with regional economic integration organizations such as the European Union and the Mercado Común del Sur (MERCOSUR) in implementing the Regulations in the countries of their respective regions.
16 Acute public health threats are collectively managed
World Health Organization Acute public health threats are collectively managed 7 April, 2017 The IHR define a risk management process where States Parties work together, coordinated by WHO, to collectively manage acute public health risks. The key functions of this global system, for States and WHO, are to: detect verify assess inform assist Timely and enhanced epidemic intelligence Real-time exchange of situational reports and other data for decision-making Enhanced information management and risk communications Joint risk analysis and decision support Action planning and coordination of response activities Technical partnerships to support international health security.
17 WHO to help countries managing events
World Health Organization WHO to help countries managing events 7 April, 2017 New WHO global Event Management System WHO Regional Alert and Response teams Train countries’ NFPs and WHO contact points for event management Expand GOARN and other specialized and regional support networks Develop new tools and standard operating procedures Carry out IHR exercises WHO wide-exercise tests the global system for international health security On, 15 June 2007, the day of the IHR’s entry into force, WHO will hold the first exercise to test its preparedness to successfully manage and support States during public health emergencies of international concern. As part of WHO’s increased responsibilities under the revised IHR, considerable effort has been made to establish procedures, technological and human resource infrastructures to ensure WHO is ready to receive, analyse and disseminate information and make recommendations for dealing with public health emergencies of international concern. The exercise on 15 June is the first of a series meant to test the mechanisms in place, their compliance with the IHR and opportunities for improvement. It consists of notifying a public health event involving multiple regions. This is an occasion to validate policy direction and coordination, information management and risk assessment capacity as well as the ability to utilize communications methods to report events from the country level through the Regional Offices to Headquarters. Participants will span many levels of WHO across the globe, including selected country office staff, WHO IHR contact points, Regional Directors, Regional Advisors, WHO Emergency and Preparedness and Response group at Headquarters, and the office of the Director-General.
18 As each country builds its capacity, the entire world wins
World Health Organization 7 April, 2017 The greatest assurance of public health security will come when all countries have in place the capacities for effective surveillance and response, for: infectious diseases radiological-related diseases chemical-related diseases food-related diseases Timeline «As soon as possible but no later than five years from entry into force» 2 years (2) + (up to 2) Each country has committed to develop and maintain core public health capacities for surveillance and response. These capacities encompass outbreaks of infectious diseases and diseases of chemical, radiological and food origin. Health services and facilities are also to be developed at important international ports, airports and ground crossings. 15 June 2007 2009 2012 2014 2016 Planning Implementation
19 Countries’ challenges for IHR implementation
World Health Organization Countries’ challenges for IHR implementation 7 April, 2017 Mobilize resources and develop national action plans Strengthen national capacities in alert and response Strengthen capacity at ports, airports, and ground crossings Maintaining strong threat-specific readiness for known diseases/risks Rapidly notify WHO of acute public health risks Sustain international and intersectoral collaboration Monitor progress of IHR implementation
20 What will WHO do under the IHR?
World Health Organization What will WHO do under the IHR? 7 April, 2017 Designate WHO IHR contact points Support States Parties in assessing their public health risks, through the notification, consultation, and verification processes Inform State Parties of relevant international public health risks Recommend adapted public health measures Assist States Parties in their efforts to investigate outbreaks and meet the IHR national requirements for surveillance and response
21 Benefit from IHR implementation
World Health Organization 7 April, 2017 Lives saved Good international image No unilateral travel and trade restrictions Public trust No political and social turmoil The legally binding nature of IHR(2005) does not include an enforcement mechanism per se for the States which fail to comply with its provisions. Nevertheless, the potential consequences of non-compliance, especially in economic terms, are a powerful compliance tool. Being a binding international agreement, the IHR(2005) are associated to greater expectations of compliance by their State Parties through greater expectations of compliance by other parties and concerns about potential retributive measures by other countries in case of non-compliance. Working together with WHO to control a public health event and to communicate accurately any associated to other States or their populations helps to protect against unjustified measures being adopted unilaterally by other States.
Презентация была опубликована 4 года назад пользователемАлександра Викторовна
Похожие презентации
Презентация на тему: » ВОЗ» — Транскрипт:
1 Приготовила : Рыбалко Александра, студентка 209 группы
3 Всемирная ассамблея здравоохранения Исполнительный комитет Секретариат
4 Всемирная ассамблея здравоохранения ( англ. World Health Assembly, сокр. Ассамблея здравоохранения ) высший руководящий орган Всемирной организации здравоохранения. Ассамблея здравоохранения собирается на ежегодные сессии, как правило в мае в Женеве во Дворце Наций ООН.
5 Исполнительный Комитет Всемирной Организации Здравоохранения руководящий орган ВОЗ, осуществляет управление организацией в перерывах между сессиями Всемирной ассамблеи здравоохранения. Состоит из 34 членов.
6 предоставление международных рекомендаций в области здравоохранения установление стандартов здравоохранения сотрудничество с правительствами стран в области усиления национальных программ здравоохранения разработка и передача соответствующих технологий, информации и стандартов здравоохранения.
World Health Organization
Published byRosaline Dalton Modified over 7 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
2 World Health Organization
Personnel World Health Organization 15 April, 2017 Objectives To review general issues related to personnel To review requirements for key personnel To review the training of personnel To consider some specific issues There are four objectives for this session: 1. We are going to start by looking at the principles and general issues around personnel such as the personnel policies and practices. 2. We will look at the responsibilities of certain key personnel in the organization. 3. We will review continuous training of personnel. 4. We will look at the specific issues that are likely to arise during your inspection visits.
3 World Health Organization
Personnel 15 April, 2017 Principle Establishment and maintenance of satisfactory system of QA, manufacture and control of products and actives rely on people. Must be sufficient qualified personnel to carry out tasks Individual responsibilities must be clearly defined and understood by individuals concerned Written job descriptions All personnel should be aware of the principles of GMP that affect them The principle that companies should apply to their people has a number of key features. The personnel is the most important asset of a company, and the easiest to neglect. The establishment and maintenance of a satisfactory system of quality assurance and GMP relies upon people who develop the system, the people who use the system and the people who examine the system to see if it has worked. People are involved, no matter how automated the process or how capital intensive the operation. The behaviour of the people is fundamental to any system of GMP. Personnel policies must reflect this. In our first group session we will be looking at those policies that encourage compliance with GMP. Sufficient number of staff must be available to carry out the work for which the manufacturer is responsible. These people must have the level of training and experience that will enable them to do their work. The staff must have written job descriptions to ensure that they understand clearly what it is that they have to do, and what they are responsible for. Finally these staff must have a knowledge and understanding of GMP to enable them to carry out their duties in accordance with GMP. Let us now look at these areas in more detail. 9.1
5 World Health Organization
Personnel 15 April, 2017 General (2) All responsible staff should have specific duties recorded in individual written job descriptions Have adequate authority to carry out responsibilities May delegate to designated deputies with qualifications No gaps or unexplained overlaps Organization chart Staff must have a clear job description which tells them and the rest of the company what their role is, what their responsibilities are and what authority they have to carry out their tasks. The company should also have a written organization chart. The combination of organization chart and written job descriptions enables the company to see quickly whether there are any gaps or whether there are any areas of overlap, owing to too many people being involved. The organisation chart should make clear and ensure the independence of QA/QC from production. Personnel involved in QA/QC must have the authority to carry out their responsibilities. This is very easy to say and sometimes not so easy to ensure. Problems can emerge in every size of company, from small private companies to very large multinational enterprises. They arise because of a combination of human interactions, and the pressures placed on people by the business considerations. 9.3
6 Basic Principles of GMP
Organization chart This is NOT what it should look like
7 World Health Organization
Personnel 15 April, 2017 General (3) All personnel should be aware of GMP Must receive training in GMP: initial training continuing training including hygiene standards Motivated to support the establishment maintain high-quality standards All personnel involved with materials and products should receive GMP training. This training should commence upon recruitment and continue throughout employment. The training should be appropriate to their needs and position within the company, and should include training in hygiene standards. Personnel policies should be designed to encourage people to support the development and maintenance of high quality standards in all work performed. 9.4
8 World Health Organization
Personnel 15 April, 2017 General (4) Prevent unauthorized access To production areas Storage areas Quality control Stop personnel who do not work in these areas using them as passageways The company should prevent people who have not been properly trained from entering any production, storage or quality control area without strict supervision. For example: it should not be the case that people from accounts are allowed to walk through the factory to get to the warehouse to pick up or deliver invoices. Access to all other areas of the company should be organized so that no entry to production, storage or laboratory areas is necessary (see Premises). 9.5
9 Basic Principles of GMP
World Health Organization 15 April, 2017 The trainer should explain ways of ensuring access control to areas. Some companies have electronic card systems that allow entry to authorized persons to specific areas, others have a code that has to be entered at the door, etc. Only people authorized to go to areas, should be allowed to enter, wearing appropriate garments. They should have the relevant training before entering the areas. IN some cases, to ensure also better control between different classes of areas, airlocks are used.
10 World Health Organization
Personnel 15 April, 2017 Key Personnel (1) Key personnel (which normally should be full-time) positions include: Authorized person Head of Production Head of Quality Control May delegate functions – not responsibility Heads of Production and Quality Control should be independent of each other We have dealt with the generalities of an organization so far. We shall now deal with the requirements for certain key people in the organization. Who are these key people? The heads of production, quality control, sales and distribution and the authorized person who releases product for sale are all key people. Normally they should all be full-time positions. The heads of production and quality control should not report to one another (although they may both report to a technical director) but may share certain responsibilities. In large organizations, it may be necessary to delegate some of the functions; however, the responsibility cannot be delegated. We will look in a minute at shared responsibilities. Different countries take a different view of reporting relationships. Legislation in each country differs slightly and, of course, local legislation must be followed. The principle is that there must be independence of quality control from production. These key personnel must have the education and experience which is appropriate to their positions. As we mentioned earlier, difficulties can arise when family members who are recruited and who are inexperienced. 9.6
11 World Health Organization
Personnel 15 April, 2017 Key Personnel (2) Should posses appropriate qualifications Scientific education such as: chemistry or biochemistry chemical engineering microbiology pharmaceutical sciences and technology pharmacology and toxicology physiology; or other related science subjects relevant to the responsibilities to be undertaken The WHO GMP text part one, section 9.7 refers to some of the acceptable qualifications of key personnel and it goes into a lot of detail. Key personnel should first of all have the educational background specified by local legislation and with the requirements set out in company policy. This will include a combination of chemistry, biochemistry, chemical engineering, microbiology, pharmaceutical sciences and technology, pharmacology and toxicology, physiology or other related science subjects relevant to the responsibilities to be undertaken. In particular, the GMP text talks about gaining experience not in a haphazard way, but under specific guidance of an expert. This is in order to equip personnel with an ability to take difficult decisions in a professional and scientific way, and to resolve the problems encountered in manufacturing and quality control. It is clear, therefore, that we are talking about professionals who have had a practical as well as an academic training. They should also continue to have training in their area of expertise. There are others who play an important role in the operation of the factory, for example, engineers. Engineers are crucial to the maintenance and operation of the facility and equipment, in particular, for the processes of planned preventative maintenance and validation. 9.7
12 World Health Organization
Personnel 15 April, 2017 Key Personnel (3) Should posses appropriate experience Practical experience Manufacture and quality assurance Preparatory period under professional guidance sometimes needed Education and experience should enable personnel to take difficult decisions in an independent, professional and scientific way resolve the problems encountered in manufacturing and QC Personnel should also posses appropriate experience including practical experience in the manufacture and quality assurance of pharmaceutical products. It is common and recommended, that there should be a preparatory period under professional guidance for new employees. Education and experience should enable personnel to take difficult decisions in an independent, professional and scientific way, and to resolve the problems encountered in manufacturing and QC. 9.7
13 World Health Organization
Personnel 15 April, 2017 Shared Responsibilities (1) Heads of Production and Quality Control may share/jointly exercise some responsibilities relating to quality: authorization of written procedures (SOPs) and other documents, including amendments monitoring and control of manufacturing environment plant hygiene process validation and calibration training, including application and principles of QA approval and monitoring of suppliers and contract acceptors The responsibilities shared by the heads of the production and quality control departments are described in the WHO GMP texts. Clear written job descriptions must be available for these positions, showing where the shared responsibilities are. It is sensible that all procedures and documents used in manufacturing are approved by both the production and quality control departments. It is most important that an effective system of change control is implemented. It is essential that as SOPs are changed, all those who use them are given the latest version. Monitoring of the manufacturing environment is a task to be shared between QA/QC and production. These departments should conduct the monitoring and testing as appropriate, with the results made available to all who need to know. Both production and quality control have a role to play in the development and maintenance of an appropriate factory sanitation and hygiene management system. Both have a major contribution to make in the validation of processes and the calibration of equipment. A comprehensive training programme is required for personnel. The production and quality control department have a role to play in the development of the training programme. The heads of these departments, therefore, have a shared responsibility for the implementation of that programme. Approval of all suppliers and contract manufacturers is also an area of shared responsibility, with each contributing his/her own particular expertise 9.8
14 World Health Organization
Personnel 15 April, 2017 Shared Responsibilities (2) Designation and monitoring of storage conditions for materials and products Performing and evaluating in-process controls Retention of records Monitoring compliance with GMP Inspection, investigation, and taking of samples to monitor factors which may affect quality Responsibility for the designation and monitoring of storage conditions is also shared. Since both have responsibility for the generation of records relevant to batches, then the arrangements for the storage of those records may be a shared responsibility. Alternatively a separate department may be available that manages all aspects of documentation and batch records. Monitoring of compliance with GMP is also a shared responsibility. This has to be correct because the achievement of GMP is everyone’s responsibility. Some aspects of inspection, investigation and sampling may be conducted by people other than QC to monitor factors that affect quality of the products. Sampling should only be done by persons trained in the methods to be used. 9.8
19 World Health Organization
Personnel 15 April, 2017 Authorized person: Responsibilities (1) Compliance with technical and regulatory requirements Approval of the release of finished product for sale Establishment and implementation of quality system Development of quality manual Supervision of self-inspections and quality audits Authorized person: Responsibilities (1) The authorized person is responsible for compliance with technical or regulatory requirements related to the quality of finished products and the approval of the release of the finished product for sale. The authorized person will also be involved in other activities, including the following: (a) implementation (and, when needed, establishment) of the quality system; (b) participation in the development of the company’s quality manual; (c) supervision of the regular internal audits or self-inspections; 9.11
20 World Health Organization
Personnel 15 April, 2017 Authorized person: Responsibilities (2) Oversight of the QC department Participation in external audits and vendor audits Participation in validation programmes May delegate approval of release of product through approved procedure Normally by QA by means of batch review The authorized person will also be involved in other activities, including the following: (d) oversight of the quality control department; (e) participation in external audit (vendor audit); (f) participation in validation programmes. The function of the approval of the release of a finished batch or a product can be delegated to a designated person with appropriate qualifications and experience who will release the product in accordance with an approved procedure. This is normally done by quality assurance by means of batch review. 9.12, 9.13
23 World Health Organization
Personnel 15 April, 2017 Training (1) Training, in accordance with a written, approved programme all personnel whose duties take them into production areas; or into control laboratories; and for others whose activities could affect the quality of the product including technical, maintenance and cleaning personnel Induction and continuing training on theory and practice of GMP and their duties training records should be kept practical effectiveness checked training before undertaking any new task Inspectors should check the company procedure, training materials and records on training provided. Training should be given in accordance with a written, approved programme to all personnel whose duties take them into production areas; or into control laboratories; and for others whose activities could affect the quality of the product including technical, maintenance and cleaning personnel) On induction and continuing; 1. Each company should establish a written training programme. New employees understand what is expected of them and the risks to patients and consumers if the products they make do not conform to requirements. This initial training must be given to all employees who have a direct impact on product quality. The training programme may consist of at least two parts. The first will be a general programme that all employees should receive which explains GMP and the importance of GMP to the company. There may well be a second programme, explaining the specific issues about the individual’s department. 2. There should be a written re-training programme for all employees to ensure that their skills are continually brought up to date and that they are introduced to changes in practice as these develop. 3. As employees go through their training, records should be kept of the training received and performance against tests. People have to realise that good performance is required otherwise retraining will be required. 4. All areas of GMP relevant to the individual must be covered. 5. Training records must be kept to ensure that as employees move around the company, they are not required to carry out work for which they have not been trained. 10.1, 10.2
25 World Health Organization
Personnel 15 April, 2017 Visitors or Untrained Personnel Preferable not to enter production and control areas. If this is unavoidable then: They must be given information in advance, particularly about personal hygiene protective clothing requirements Must be accompanied and closely supervised at all times One of the questions that you should raise in all companies is how they handle visitors. The answer should be that their presence should be treated as a potential risk to the product and, therefore, steps should be taken to ensure that they cannot cause any hazard to product quality. Ideally, the company will do this by factory design that ensures that visitors cannot gain access to areas in operation. This is usually difficult and so it is necessary to give visitors a full briefing and to provide them with full protective clothing and give them strict instructions about where they may stand. For visitors there can be no exceptions to the rule of the wearing of appropriate clothing to protect the product. A word at this stage about visits by the owners of the business. This may be a difficult area because owners may feel that if the company is theirs they may do what they want, when they want. Somehow they have to understand that their investment is at risk if they do not behave as necessary. (Use flipchart again to record comments from the audience). 10.5
26 World Health Organization
Personnel 15 April, 2017 Consultants and contract staff Should be qualified for the services provided Training records maintained Records should prove qualifications A manufacturer may use the services of a consultant and contract staff. The company has to ensure that these people are qualified for the services provided. It may be useful for you to review also the training records maintained for contract staff. Some companies use contract workers for some activities including packaging of products. They should have been properly trained in GMP, QA and the relevant procedures and processes that they are responsible for. Records should prove persons’ qualifications 10.6
27 World Health Organization
Personnel World Health Organization 15 April, 2017 Group Session What do you think will be the key personnel issues to arise during an inspection? What sort of responses do you think you should give to these issues when they become apparent? Having heard the presentation on personnel, use what you know to develop thoughts on the likely issues regarding the organization of personnel that you will meet during inspections in your countries. More personal issues arising during an inspection are dealt with in the modules on GMP inspections. Once you have drawn up a list, develop the responses that you feel would be appropriate in those circumstances.
28 World Health Organization
Personnel World Health Organization 15 April, 2017 Possible Issues – I Limited number of staff Inadequate qualifications Inadequate experience Owner interferes in quality decisions Lack of means to develop training materials In a company the issues will be around a lack of skills and resources to comply with all the requirements of GMP: Limited staff numbers may mean that people are under pressure to perform. They may be trying to do too much. There may be a lack of deputies during times of illness or holidays. Recruited staff may have inadequate qualifications. Recruited staff may have inadequate experience or experience in an inappropriate area. Sometimes the owner recruits relatives who are inadequately qualified or experienced. The owner may interfere with quality decisions, particularly if orders are required urgently or are very valuable. Senior staff may have difficulty in combating this, since it may cost them their jobs. Smaller companies may have no means to develop training materials to educate their staff in the requirements of GMP. They do not become members of the local manufacturers’ association because of cost. They do not then have access to training programmes that are available through the association.
29 World Health Organization
Personnel World Health Organization 15 April, 2017 Possible Issues – II Company procedures take precedence over local legislation Unclear organization diagram Staff movement Inadequate training records Illness Subsidiaries of multinational companies may claim that company procedures or standards take precedence over local legislation. If this is claimed, it will be most unusual since all multinationals require local companies to conform first to local legislation. It will be worth exploring with the company what benefits are obtained by not conforming to local legislation. Large organizations often move people around through promotion, training, recruitment or relocation. In so doing they can lose sight of the requirements of GMP. Managers can be promoted into positions for which they are not qualified or experienced. Companies may not keep adequate training records even though people are apparently undergoing training. As with small companies, large companies may have personnel policies that penalize people. The problem is that if people are not going to be paid when sick or injured, they may work on under circumstances that create a risk to the product. What happens when they have an open wound, for example?
World Health Organization
Published byLisa Randall Modified over 7 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
19 April, 2017 Supplementary Training Modules on Good Manufacturing Practice Validation In this supplementary training module, we will be looking at the recommendations by WHO, on Validation and qualification. The module consists of 7 parts: Part 1. General overview on qualification and validation Part 2. Qualification of HVAC and water systems Part 3. Cleaning validation Part 4. Analytical method validation Part 5. Computerized system validation Part 6. Qualification of systems and equipment Part 7. Non sterile product process validation Each part deals with a specific topic, and each part can be presented in about one to one and a half hours time. Presenters should know the topics and add practical examples to the texts taken from the WHO guideline. WHO Technical Report Series, No. 937, Annex 4.
2 World Health Organization
19 April, 2017 Validation Part 1. General overview on qualification and validation Part 2. Qualification of HVAC and water systems Part 3. Cleaning validation Part 4. Analytical method validation Part 5. Computerized system validation Part 6. Qualification of systems and equipment Part 7. Non sterile product process validation
3 World Health Organization
19 April, 2017 Supplementary Training Modules on Good Manufacturing Practice Non sterile product process validation Part 7 WHO Technical Report Series, No. 937, Annex 4. Appendix 7.
4 World Health Organization
Validation World Health Organization 19 April, 2017 Objectives To discuss non-sterile process validation, focusing on: General recommendations Prospective validation Concurrent validation Retrospective validation Revalidation Change control Objectives To discuss Non-sterile process validation, focusing on: General recommendations Prospective validation Concurrent validation Retrospective validation Revalidation Change control
5 World Health Organization
Validation 19 April, 2017 Principle Documented evidence: Process is capable of reliably and repeatedly rendering a product of the required quality Planning, organizing and performing process validation Process validation protocols Data collected and reviewed against predetermined acceptance criteria – recorded in validation report Principle Documented evidence: Process is capable of reliably and repeatedly rendering a product of the required quality. Planning, organizing and performing process validation Process validation protocols Data collected and reviewed against predetermined acceptance criteria – recorded in validation reports. 1.1 – 1.2
6 World Health Organization
Validation 19 April, 2017 Scope General aspects of process validation for the manufacture of non-sterile finished products Should cover at least the critical steps and parameters, i.e. those that may have an impact on the quality of the product 2. Scope 2.1 These guidelines describe the general aspects of process validation for the manufacture of non-sterile fi nished products. 2.2 Normally process validation should cover at least the critical steps and parameters (e.g. those that may have an impact on the quality of the product) in the process of manufacturing a pharmaceutical product.S 2.1 – 2.2
8 World Health Organization
Validation 19 April, 2017 Prospective validation Critical factors or parameters possibly affecting finished product quality to be identified during product development Breakdown of production process into individual steps Evaluate each step Determine the criticality of these factors through a “worst-case” challenge where possible 4. Prospective validation 4.1 Critical factors or parameters that may affect the quality of the finished product should be identified during product development. To achieve this, the production process should be broken down into individual steps, and each step should be evaluated (e.g. on the basis of experience or theoretical considerations). 4.2 The criticality of these factors should be determined through a “worst-case” challenge where possible. 4.1 – 4.2
9 World Health Organization
Validation 19 April, 2017 (continued) Prospective validation protocol should include: description of the process and of the experiment equipment and/or facilities to be used including measuring or recording equipment (and its calibration status) variables to be monitored details of the samples to be taken product performance characteristics/attributes to be monitored, together with the test methods acceptable limits and time schedules personnel responsibilities details of methods for recording and evaluating results, including statistical analysis 4.3 Prospective validation should be done in accordance with a validation protocol. The protocol should include: — a description of the process; — a description of the experiment; — details of the equipment and/or facilities to be used (including measuring or recording equipment) together with its calibration status; — the variables to be monitored; — the samples to be taken — where, when, how, how many and how much (sample size); — the product performance characteristics/attributes to be monitored, together with the test methods; — the acceptable limits; — time schedules; — personnel responsibilities; and — details of methods for recording and evaluating results, including statistical analysis. 4.3
10 World Health Organization
Validation 19 April, 2017 Approach: Equipment, production environment and analytical testing methods – already fully validated e.g. during installation qualification and operational qualification Appropriately trained personnel and batch manufacturing documentation prepared after these critical parameters have been identified, and machine settings, component specifications and environmental conditions have been determined and specified 4.4 All equipment, the production environment and analytical testing methods to be used should have been fully validated (e.g. during installation qualification and operational qualification). 4.5 Personnel participating in the validation work should have been appropriately trained. 4.6 Batch manufacturing documentation to be used should be prepared after these critical parameters of the process have been identified, and machine settings, component specifications and environmental conditions have been determined and specified. 4.7 A number of batches of the final product should then be produced. The number of batches produced in this validation exercise should be sufficient to allow the normal extent of variation and trends to be established and to provide sufficient data for evaluation.S 4.4 – 4.6
11 World Health Organization
Validation 19 April, 2017 Approach (2) A number of batches of the final product should then be produced What number of batches? sufficient to allow the normal extent of variation and trends to be established and to provide sufficient data for evaluation Data within the finally agreed parameters from at least three consecutive batches, giving product of the desired quality may be considered acceptable 4.7 A number of batches of the final product should then be produced. The number of batches produced in this validation exercise should be sufficient to allow the normal extent of variation and trends to be established and to provide sufficient data for evaluation.S 4.7 – 4.8
12 World Health Organization
Validation 19 April, 2017 Approach (3) Same size batches Full-scale production batch size If not possible – reduced batch size considered Validity of assumptions made should be demonstrated when full-scale production starts Extensive testing at various stages in the manufacturing process – including on the final product and its package 4.9 The batches should be of the same size, and should be the same as the batch size intended in full-scale production. Where this is not possible, the reduced batch size should be considered in the design of the protocol and when full-scale production starts, the validity of any assumptions made should be demonstrated. 4.10 Extensive testing should be performed on the product at various stages during the manufacturing process of the batches, including on the final product and its package. 4.9 – 4.10
13 World Health Organization
Validation World Health Organization 19 April, 2017 Setting Limits: may include Marketing authorization limits stability specifications Release specification Validation limits Setting limits: Marketing authorization limits: usually the national compendia limits or those agreed at the time of product registration. The product must meet these at any time that it is on the market and within its expiry date. Stability specifications: The specification minimally needed to maintain required potency over the shelf life of the product, based on stability study data. Release specification: the product must meet at the time of release and in order to allow for any changes (super-potency, sub-potency, dissolution, disintegration, etc) over shelf life of product. This is the simplest criteria for setting validation acceptance testing, but will not necessarily include process capability. In the development of acceptance criteria, all three of the above specification areas must be taken into consideration, including an analysis of data gathered during the initial development and stability work. In most cases, this data will be limited but will give enough information on test and process variability to allow for some guidance. The most important thing to remember is to keep the statistics simple. Validation acceptance criteria may be tighter than, or equal to the release limits, which may be tighter than, or equal to the compendial limits. Marketing authorization limits based on stability specifications Batch release limits Validation limits
15 World Health Organization
Validation 19 April, 2017 Determining critical control points A useful strategy to determine which steps to study intensively, is to “flow chart” the process and conduct a hazard analysis of critical control points. Critical control points indicate critical processing steps. It is necessary to note how often critical control points come at the end stages as the value-adding process proceeds. The flow chart above shows a tablet granulation process where Step XVI and XVIII have been identified as a critical control point. Blend uniformity and cleaning validation has to be performed at step XVI, and during actual manufacture, a reconciliation of the actual yield against the expected yield must be performed before the tablet compression step. (The trainer should explain that this diagram is incomplete, as there are other IQ, OQ, PQ requirements, steps, etc. to be included The slide provides an example only.)
17 World Health Organization
19 April, 2017 Validation Solid dose mixing (2) In situ analysis Methods of analysis Statistical analysis inter-batch intra-batch within-sample-site Solid dose mixing: (Contd) There are now manyin situ spectroscopic approaches, such as infrared (IR), near-infrared (NIR), and Raman spectroscopy, which are fast, accurate and easily performed. Probes may be placed directly into the mixing vessel or be positioned at windows along the walls of the vessels, allowing for real-time, uninterrupted homogeneity measurements. Remember uniformity or homogeneity are being considered, not a determination of the active. Although the best marker is the active (must be the active for low dose and potent product), the marker can be chosen if it is representative of the blend. The methods of analysis for these samples of the blend are extracted and assayed by UV or HPLC, or similar validated test method. Note that non-specific methods are satisfactory – which is a big difference from e.g. stability studies. The actual spectra (from, for example, near-Infrared) and methods of calculating the homogeneity of the actives should be subject to statistical analysis. The within-sample-site variability must also be acceptable for low dilution powders, such as micro-dose tablets or capsules. This can usually be demonstrated on just the first batch, not for each of the three batches. It is a “minivalidation” of the sampling thief and sampling method used.
21 World Health Organization
Validation 19 April, 2017 Results in the report that includes, e.g. process description including details of critical steps detailed summary of the results obtained from in-process and final testing, including data from failed tests raw data or reference to these any work done in addition to that specified in the protocol any deviations from the protocol with an explanation a review and comparison of the results with those expected formal acceptance or rejection of the work by the team or persons designated as being responsible for the validation, after completion of any corrective action or repeated work 4.11 The results should be documented in the validation report. As a minimum, the report should include: • a description of the process: batch/packaging document, including details of critical steps; • a detailed summary of the results obtained from in-process and final testing, including data from failed tests. When raw data are not included, reference should be made to the sources used and where it can be found; • any work done in addition to that specified in the protocol, or any deviations from the protocol should be formally noted along with an explanation; • a review and comparison of the results with those expected; and • formal acceptance or rejection of the work by the team or persons designated as being responsible for the validation, after completion of any corrective action or repeated work. 4.11
22 World Health Organization
Validation 19 April, 2017 Conclusion and recommendation: Made on the basis of the results obtained Incorporated into the batch manufacturing and batch packaging documents and/or standard operating procedures (SOPs) for routine use Limits and frequencies of testing and monitoring should be specified. Actions in case of OOL If validation batches are to be sold or supplied: manufactured under GMP conditions Compliance with the marketing authorization 4.12 A conclusion and recommendation should be made on the extent of monitoring and the in-process controls necessary for routine production, on the basis of the results obtained. 4.13 The conclusion and recommendation should be incorporated into the batch manufacturing and batch packaging documents and/or standard operating procedures (SOPs) for routine use. Limits and frequencies of testing and monitoring should be specified. Actions to be taken in the event of the limits being exceeded should be specified. 4.14 Batches manufactured as part of the validation exercise, and intended to be sold or supplied, should have been manufactured under conditions that comply fully with the requirements of good manufacturing practice and the marketing authorization (where applicable). 4.12. – 4.14.
23 World Health Organization
Validation 19 April, 2017 Concurrent validation May be appropriate to validate a process during routine production Can you give any examples? Decision made by appropriately authorized personnel Premises and equipment previously qualified Done as per validation protocol; and results documented in the validation report Concurrent validation 5.1 In certain cases, it may be appropriate to validate a process during routine production, e.g. where the product is a different strength of a previously validated product, a different tablet shape or where the process is well understood. 5.2 The decision to carry out concurrent validation should be made by appropriately authorized personnel. 5.3 It is essential that the premises and equipment to be used during concurrent validation have been previously qualified. 5.4 Prospective validation should be done in accordance with a validation protocol. 5.5 The results should be documented in the validation report. 5.1 – 5.5
24 World Health Organization
Validation 19 April, 2017 Retrospective validation Comprehensive review of historical data Requires a protocol and a report with a conclusion and a recommendation Not the preferred method of validation, and used in exceptional cases only: e.g. for well-established processes Inappropriate in case of changes (e.g. equipment) Retrospective validation 6.1 Retrospective validation is based on a comprehensive review of historical data to provide the necessary documentary evidence that the process is doing what it is believed to do. This type of validation also requires the preparation of a protocol, the reporting of the results of the data review, a conclusion and a recommendation. 6.2 Retrospective validation is not the preferred method of validation and should be used in exceptional cases only. It is acceptable only for well-established processes and will be inappropriate where there have been changes in the composition of the product, operating procedures or equipment. 6.1 – 6.2
25 World Health Organization
Validation 19 April, 2017 Retrospective validation (2) Sufficient data to be reviewed to provide a statistically significant conclusion Satisfactory results of retrospective validation only serve as an indication that the process does not need to be subjected to validation in the immediate future 6.3 Sufficient data should be reviewed to provide a statistically significant conclusion. 6.4 When the results of retrospective validation are considered satisfactory, this should serve only as an indication that the process does not need to be subjected to validation in the immediate future. 6.3 – 6.4
26 World Health Organization
Validation 19 April, 2017 Revalidation Standard processes (with conventional equipment) data review similar to retrospective validation Points to be considered: the occurrence of any changes in the master formula, methods, starting material manufacturer, equipment and/or instruments calibrations and preventive maintenance carried out standard operating procedures (SOPs) cleaning and hygiene programme 7. Revalidation Note: see main text on “Validation”. The need for periodic revalidation of non-sterile processes is considered to be a lower priority than for sterile processes. 7.1 In the case of standard processes using conventional equipment, a data review similar to that which would be required for retrospective validation may provide an adequate assurance that the process continues to be under control. The following points should also be considered: — the occurrence of any changes in the master formula, methods, starting material manufacturer, equipment and/or instruments; — equipment calibrations and preventive maintenance carried out; — standard operating procedures (SOPs); and — cleaning and hygiene programme. 7.1
27 World Health Organization
Validation 19 April, 2017 Change control In case of changes, consider the change and its impact on the process validation Examples of changes (requiring revalidation): manufacturing process (e.g. mixing times, drying temperatures) equipment (e.g. addition of automatic detection systems) production area and support system changes transfer of processes to another site unexpected changes (e.g. those observed during self-inspection or during routine analysis of process trend data) Change control 8.1 Products manufactured by processes that have been subjected to changes should not be released for sale without full awareness and consideration of the change and its impact on the process validation. 8.2 Changes that are likely to require revalidation may include: — changes in the manufacturing process (e.g. mixing times, drying temperatures); — changes in the equipment (e.g. addition of automatic detection systems); — production area and support system changes (e.g. rearrangement of areas or a new water treatment method); — transfer of processes to another site; and — unexpected changes (e.g. those observed during self-inspection or during routine analysis of process trend data). 8.1 – 8.2
28 World Health Organization
Validation 19 April, 2017 Suspensions Syrups Non sterile products Capsules Creams Ointments Tablets
29 World Health Organization
Validation 19 April, 2017 Non sterile products List some of the key parameters to be considered in the process validation of the dosage forms mentioned 2.4
30 World Health Organization
Validation 19 April, 2017 Prospective validation SUMMARY Ask te participants about principle elements of the topic on the slide
31 World Health Organization
Validation World Health Organization 19 April, 2017 Prospective validation SUMMARY Concurrent validation Ask te participants about principle elements of the topic on the slide
32 World Health Organization
Validation World Health Organization 19 April, 2017 Prospective validation SUMMARY Concurrent validation Retrospective validation Ask te participants about principle elements of the topic on the slide
33 World Health Organization
Validation 19 April, 2017 Prospective validation SUMMARY Concurrent validation Revalidation Retrospective validation Ask te participants about principle elements of the topic on the slide
34 World Health Organization
Validation 19 April, 2017 Prospective validation SUMMARY Concurrent validation Revalidation Retrospective validation Ask te participants about principle elements of the topic on the slide Change control
35 World Health Organization
Validation World Health Organization 19 April, 2017 Group Session You are given a tablet manufacturing flow chart to study List the critical steps that are required to be validated List the critical equipment required to be qualified Identify the variables and construct a table as directed In this group session, (see handout ) you should list the aspects that you will evaluate when assessing the validation for the project that your group had been given. Identify the critical parameters that should have been evaluated by the manufacturer. List the tests to be carried out and comment on the acceptance criteria to be set.
Презентация на тему: Всемирная Организация Здравоохранения
Всемирная Организация Здравоохранения
Всеми рная организа ция здравоохране ния (ВОЗ, англ. World Health Organization, WHO) — специальное агентство Организации Объединённых Наций, состоящее из 193 государств-членов, основная функция которого лежит в решении международных проблем здравоохранения и охране здоровья населения мира. Она была основана в 1948г. со штаб-квартирой в Женеве в Швейцарии. Всеми рная организа ция здравоохране ния (ВОЗ, англ. World Health Organization, WHO) — специальное агентство Организации Объединённых Наций, состоящее из 193 государств-членов, основная функция которого лежит в решении международных проблем здравоохранения и охране здоровья населения мира. Она была основана в 1948г. со штаб-квартирой в Женеве в Швейцарии.
Структура ВОЗ 1. Всемирная ассамблея по здравоохранению. 2. Исполнительный комитет. 3. Секретариат. Всемирная ассамблея здравоохранения является высшим органом ВОЗ, принимающим решения. Основное внимание на ее сессиях, в работе которых участвуют делегации из всех государств-членов ВОЗ, сосредоточено на повестке дня в области здравоохранения, подготовленной Исполнительным комитетом. Основными функциями Ассамблеи являются определение направления деятельности Организации, назначение Генерального директора, наблюдение за финансовой деятельностью Организации, пересмотр и утверждение проекта программного бюджета. Ее ежегодные сессии проводятся в Женеве, Швейцария. Исполнительный комитет состоит из 34 членов, технически квалифицированных в области здравоохранения, каждый из которых назначается государством-членом, избранным для этого Всемирной ассамблеей здравоохранения. Государства-члены избираются сроком на три года. Комитет проводит не менее двух сессий в год; основная сессия обычно проводится в январе, а другая, менее продолжительная сессия, проводится в мае, сразу же после Ассамблеи здравоохранения. Основными функциями Исполнительного комитета является проведение в жизнь решений и политики Ассамблеи здравоохранения, оказание ей консультативной помощи и общее содействие ее работе.
Задачи ВОЗ Предоставление международных рекомендаций в области здравоохранения Установление стандартов здравоохранения Сотрудничество с правительствами стран в области усиления национальных программ здравоохранения Разработка и передача соответствующих технологий, информации и стандартов здравоохранения.
Сферы деятельности ВОЗ Укрепление и совершенствование национальных служб здравоохранения; Предупреждение неинфекционных и инфекционных заболеваний и борьба с ними; Охрана и оздоровление окружающей среды; Охрана здоровья матери и ребёнка; Подготовка медицинских кадров; Развитие медико-биологических исследований; Санитарная статистика.
Региональные бюро ВОЗ Европейское — в Копенгагене (Дания), Американское — в Вашингтоне (США), Восточно-Средиземноморское (Средиземноморское) — в Каире (Египет), Юго-Восточной Азии (Азиатское) — в Дели (Индия), Западной части Тихого океана (Тихоокеанское) — в Маниле (Филиппины), стран Африки южнее Сахары (Африканское) — в Браззавиле (Конго).
Работа ВОЗ в настоящее время В настоящее время приоритетными направлениями являются: Развитие систем здравоохранения в странах в соответствии с резолюцией об основных принципах национального здравоохранения (1970), в которой чётко обозначены ответственность государства, средства профилактики, участие населения, использование достижений науки и т. д.; Подготовка и усовершенствование кадров здравоохранения; Развитие первичной медико-санитарной помощи в соответствии с Алма-Атинской декларацией ВОЗ-ЮНИСЕФ (1978); Охрана и укрепление здоровья различных групп населения; Охрана окружающей среды; Борьба с инфекционными и паразитарными болезнями, иммунизация и вакцинация против основных эпидемических заболеваний; Охрана и укрепление психического здоровья; Обеспечение здоровья матери и ребёнка; Информирование по проблемам охраны здоровья; Расширенная программа научных медицинских исследований; Актуальные направления консультативной и технической помощи странам-членам.
World Health Organization
Published bySeamus Gale Modified over 8 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
31 March, 2017 Supplementary Training Modules on Good Manufacturing Practice Heating, Ventilation and Air- Conditioning (HVAC) Part 2: HVAC systems and components Section 7
2 World Health Organization
31 March, 2017 HVAC Objectives In the following slides, we will study the components of air- handling systems in order to: Become familiar with the components Know their functions Become aware of possible problems Objectives: For you, as inspectors, to be able to judge whether the air handling systems which you encounter during your factory inspections are adequate or not, it is necessary to know how such systems work, and to be aware of what problems may arise in terms of the components of the system. Therefore, the objectives of this part of module 3 are to study the components of air handling systems in order to: Become familiar with the components Know their functions Become aware of possible problems
3 World Health Organization
HVAC 31 March, 2017 General Design of HVAC is dependent on required degree of air cleanliness Suitable components should be selected including: fans, driers, filters, ducts, grilles, etc. 7. HVAC systems and components Note: The required degree of air cleanliness in most OSD manufacturing facilities can normally be achieved without the use of high-effi ciency particulate air (HEPA) fi lters, provided the air is not recirculated. Many open product zones of OSD form facilities are capable of meeting ISO Class 8, “at-rest” condition, measured against particle sizes of 0.5 μm and 5 μm, but cleanliness may not be classifi ed as such by manufacturers. 7.
4 World Health Organization
31 March, 2017 HVAC Main subsystems + Production Room Exhaust air treatment Central air handling unit Terminal air treatment at production room level Fresh air treatment (make-up air) To understand the air handling systems, it is necessary to know what their components are. A conventional Air Handling System has 4 sub-systems: 1. Air handling of the incoming (fresh) air: elimination of coarse contaminants and protection from frost if necessary. In the case of air re-circulation, the fresh air is also called make-up air. 2. Central air handling unit (AHU), where the air will be conditioned (heated, cooled, humidified or de-humidified and filtered), and where fresh air and re-circulated air, if any, (indicated here by the dotted line) will be mixed. 3. Air handling in the rooms under consideration (pressure differential system, additional filtration, air distribution). 4. Air exhaust system (filtration).
5 World Health Organization
HVAC 31 March, 2017 Components Components in HVAC may include, depending on need: Filters Fans no fan failure; including supply air fans, return air fans, exhaust air fan, dust extract system fans Driers Drying of air with chemical driers, e.g. rotating desiccant wheel Frost coils for preheating air 7.1 General 7.1.1 There should be no failure of a supply air fan, return air fan, exhaust air fan or dust extract system fan. Failure can cause a system imbalance, resulting in a pressure cascade malfunction with a resultant airfl ow reversal. 7.1.2 A schematic diagram of the airfl ow for a typical system serving a low humidity suite is represented in Fig. 23. 7.1.3 Air should be dried with a chemical drier (e.g. a rotating desiccant wheel which is continuously regenerated by means of passing hot air through one segment of the wheel). 7.1.4 The fi gure illustrates the chemical drier handling part of the fresh air/return air mixture on a by-pass fl ow. The location of the chemical drier should be considered in the design phase. Examples of appropriate locations include: — full fl ow of fresh/return air; — partial handling of fresh/return air (by-pass airfl ow); — return air only; — fresh air only; or — pre-cooled air with any of the above alternatives. 7.1.5 Possible additional components that may be required should be considered depending on the climatic conditions and locations. These may include items such as: — frost coils on fresh air inlets in very cold climates to preheat the air; — snow eliminators to prevent snow entering air inlets and blocking airfl ow; — dust eliminators on air inlets in arid and dusty locations; — moisture eliminators in humid areas with high rainfall; and — fresh air pre-cooling coils for very hot or humid climates. 7.1.6 Appropriate alarm systems should be in place to alert personnel if a critical fan fails. 7.1.7 Low-level return or exhaust air grilles are usually preferred. However, where this is not possible, a higher air change rate may be needed to achieve a specifi ed clean area classifi cation, e.g. where ceiling return air grilles are used. 7.1.1 – 7.1.7
6 World Health Organization
HVAC 31 March, 2017 Components Components in HVAC may include, depending on need: Snow eliminators Dust eliminators Moisture eliminators Precooling coils Alarm systems, grilles/diffusers, etc. 7.1.1 – 7.1.7
7 World Health Organization
31 March, 2017 HVAC Filter Silencer Terminal filter Weather louvre Control damper Fan Flow rate controller Humidifier Heating coil Cooling coil with droplet separator Production Room Overview components + Prefilter Exhaust Air Grille Heater Another way to look at an air handling system is to consider the different components and to know their function. Some of the components, particularly the filters, are essential to ensure the quality of the air. We will later consider individual components in detail. Of course, a well-designed air handling system must not only be properly designed, but also properly installed, qualified and maintained (sealed ducts, tight filters). (The trainer should make the audience aware that this slide is just an example, and that all components may not necessarily be present in each system.) Secondary Filter Recirculated air
8 World Health Organization
31 March, 2017 HVAC Components (1) Weather louvre Silencer Flow rate controller Control damper To prevent insects, leaves, dirt and rain from entering To reduce noise caused by air circulation Automated adjustment of volume of air (night and day, pressure control) Fixed adjustment of volume of air A typical HVAC unit consists of a small number of elements only. It is important that these elements are compatible, properly installed, and fulfilling their goal. Whereas a weather louvre and silencer are less critical elements, the components associated with the flow rate control are essential, as they allow adjustment of the air volumes supplied to the rooms, which in turn forms the base for a pressure differential concept: to have an automated or a fixed system is largely a financial matter, but a fixed system is more difficult to set up. Silencer – check internal lining material of silencer as this can cause contamination.
9 World Health Organization
31 March, 2017 HVAC Components (2) Heating unit Cooling unit/ dehumidifier Humidifier Filters Ducts To heat the air to the proper temperature To cool the air to the required temperature or to remove moisture from the air To bring the air to the proper humidity, if too low To eliminate particles of predetermined dimensions and/or microorganisms To transport the air Heating and cooling units (batteries), as well as humidifiers are used to adjust the climate in the room (temperature and humidity). Special de-humidifiers, on a dessiccant base, will be addressed later. Filters are one of the main components, as they determine the size of airborne particles that pass through them, and thus the hygiene class. It is wise to protect the finer filters by pre-filters, thus extending their life cycles, and making them less prone to clogging. Ducts transport the air from the air handling units to and from the rooms. Inspectors must verify that ducts do not have internal insulation as this is a great source of contamination.
10 Control damper for airflow
World Health Organization 31 March, 2017 HVAC Air-handling unit Control damper for airflow Humid room air Air heater Regeneration air Adsorber wheel Dry air Dampers to control pressure differentials are important. They can be automated or fixed. As filters get dirty the system pressure losses increase, and if airflow is not regulated, the flow decreases and pressure differentials change. This could cause flow reversal and cross-contamination. Variable speed drives for fan motors are also commonly used to control airflow. In some cases, it is necessary to have very dry air for galenical reasons in certain rooms (production of effervescent tablets and humidity sensitive products in general). To generate dry air, the air supplied to the production is passed over an adsorbant (silicagel, lithium chloride, etc.) where the humidity is removed from the air. The adsorbant is then re-generated, on a continuous or on a batch-wise base. AHU with fan Variable Speed Controller Filter Pressure Gauges De-humidification
11 Humidifier Silencer Heating and cooling units
World Health Organization 31 March, 2017 HVAC Humidifier Silencer Heating and cooling units This slide shows additional elements of the air handling units. For humidification purposes, especially in clean areas, high purity water should be used, to avoid contamination. The silencer is not important from a GMP point of view, but from an environmental one, as ventilation units can be very noisy. Be sure that the silencers are manufactured of suitable materials as the linings of standard silencers can contaminate air with particulates. Depending on the local legislation, the installation of silencers can be mandatory. The cooling unit is important during the hot season. Be aware that stagnating water (condensed water) can bring bacterial growth, which can contaminate the filters, pass through them (depending on their retention properties) and end up contaminating production areas. It is essential that there is no stagnating water. Cooling coils can be sanitized as well. Do remember that, if filters are not properly maintained, micro-organisms may grow through the filters and be carried towards the production rooms.
13 World Health Organization
31 March, 2017 HVAC HEPA or tertiary filter Primary panel filter This slide shows Primary Panel filters, which are used mainly for lower filtration efficiency or as pre-filters Secondary filters, consisting of mini-pleated media or filter bags, and is used for higher filtration efficiency. HEPA or tertiary filters, usually being the final filter in the system, providing the highest filtration efficiency. Though there is a strong relationship between filter efficiency and cleanroom class, a filter of a high efficiency does not guarantee a high cleanroom class, as many other elements play a role, such as Air flow (how the air is extracted, how well the room is “flushed”) Air speed and number of air changes Positions of air terminals Layout and presence of objects Personnel and clothing Equipment (not all machines are designed to operate in a clean environment!) Proper installation and proper maintenance Secondary filter
14 World Health Organization
31 March, 2017 HVAC This table gives an idea of the efficiency of the filters, calculated across the entire surface (integral value) or in particular spots (local value). Referring to filter ratings by percent efficiency is misleading, as there are so many different types of tests that give different efficiencies for the same filter. This can be very confusing and it is better to refer to the Committee of European Normalisation (EN) test rating i.e. G4, F8, H12, etc.
15 World Health Organization
31 March, 2017 HVAC Positioning of filters (1) AHU mounted final filter Filter in terminal position HEPA Filter + Production Room Production Room In some of the previous slides, we have seen filters both in the central air handling units ( AHU ) and terminally mounted at the production rooms. The filtered air entering a production room can be coming from: an air-handling unit, equipped with pre-filtration and the main (HEPA) filter, but at some distance from that room (left drawing); an air-handling unit, equipped with pre-filtration in the AHU, and an additional filter (HEPA) situated immediately on the air outlet (right drawing). In many cases, there are only filters in the AHU. However, for injectables and sterile forms, it is recommended that they be placed in terminal position, though there is a growing tendency to have terminal filters in all rooms where open products are handled. It is recommended that classes A & B (ISO 4, 5 & 6) have terminal HEPA filters. (Refer to: WHO Export Committee on Specifications for Pharmaceutical Preparations. Thirty-second Report. Geneva, World Health Organization, 1992:59-60 (Technical Report Series, No. 823). Annex 1, 17.3.) If we look at the advantages and disadvantages of terminal or non-terminal filters, we can say that generally speaking, the terminal positioning is more expensive; provides a better protection (any problem arising from the ducts is eliminated); is the preferred method in cleanroom classes with high requirements. HEPA Filter
16 World Health Organization
31 March, 2017 HVAC Positioning of filters (2) Prefilter AHU Main filter Ceiling exhausts Filters can be in different positions, when one considers the central AHU and the rooms. This slide shows an HVAC installation feeding 3 rooms, each one with terminal filters, all filters protected by a remote pre-filter. Room 1 has a turbulent air flow, with low level exhaust. Room 2 has a uni-directional (laminar) air flow over the largest part of the surface, hence the large number of filters. Room 3 has a turbulent air flow, with ceiling exhaust. 2 3 1 Low level exhausts
17 World Health Organization
31 March, 2017 HVAC AHU Prefilter Final filter 2 1 Positioning of filters (3) This slide shows an HVAC installation feeding two rooms, each one without terminal filters, but with remote final filters protected by a pre-filter. Room 1 has a turbulentair flow, with low level exhaust. Room 2 has a turbulent air flow, with ceiling exhaust. If there is no filter in terminal position, it should be ascertained that there are no elements between the main filter and the air outlets which could add contamination. No elements such as fans, heating/cooling batteries, should be situated downstream of the final filter.
18 Swirl Type air diffusors with terminal filters
World Health Organization 31 March, 2017 HVAC 1 2 3 4 The air flows into the rooms via so-called registers (diffusors), which are built and installed in such a way that the air is distributed evenly. Machinery or furniture can block the passage of air from the register to the exhaust point, creating unflushed zones, where counts of particles and micro-organisms could be higher. It is therefore important to consider the content of a clean room, when planning the HVAC system. In many cases, the terminal filter panel and diffusors are incorporated into one unit. It is also important that the air diffuser supplies air evenly and does not induce the circulation of dust in the room – as illustrated by the next slide. Swirl Type air diffusors with terminal filters 1 Filter 2 Tightening frame 3 Register outlet 4 Screw fixation for register
19 World Health Organization
31 March, 2017 HVAC The diffuser on the left is a normal office type diffuser which induces a lot of air to rise vertically from the floor towards the ceiling. The rising induced air has the potential for carrying a lot of dust upwards which is then spread throughout the room with the air supply. This type of diffuser readily spreads contaminants in the room and should be avoided. The preferred type of diffuser for cleanroom applications is the swirl diffuser, or perforated plate diffuser. These types do not promote the spread of dust within the room. High induction office type diffusor (avoid) Low induction swirl diffusor (preferred)
20 Regulation of room pressure – pressure differentials concept
World Health Organization 31 March, 2017 HVAC Regulation of room pressure – pressure differentials concept One of the main points in the design of production rooms is the pressure differentials concept. Pressure differentials must be defined, monitored and alarmed in critical cases. The overpressure of each room is measured against a reference point in the factory (point zero). As discussed earlier, the pressure regulation can be fixed or automated. Pressure control can be by means of automatic air flow control dampers (as shown in slide 15) or by means of fan speed control. Whatever the means used it is important to ask the manufacturer how they ensure that the pressure cascade is maintained as the filters get dirty. In the following slides, we are going to see that an overpressure concept can be very different for sterile products and for solid products. Room pressure gauges Room pressure indication panel
21 World Health Organization
31 March, 2017 HVAC Problems with components Flow rate controller Control damper Humidifier Cooling battery Filters Ducts Blocked Poorly adjusted, bad pressure differential system Bad water/steam quality/ poor drainage No elimination of condensed water/ poor drainage Incorrect retention rate/damaged/badly installed Inappropriate material/internal insulator leaking Problems may arise with components, with the following consequences: Flow rate controller Blocked No control of pressure differentials Control damper Poorly adjusted Bad pressure differential systems Humidifier Bad water/ Risks of microbial contamination steam quality Cooling Unit No elimination Risks of microbial contamination of condensed water Filters Incorrect retention Risks of contamination rate (particles, micro-organisms) Damaged Filter integrity fails Badly installed Risks of contamination (particles, micro-organisms) Ducts Inappropriate material Danger of corrosion Leaking duct work Intake of unfiltered air Internal insulation Inability to properly clean
22 World Health Organization
HVAC World Health Organization 31 March, 2017 In the next slides Consider different air types, e.g.: Supply air Return air (recirculated air) Fresh air (make-up air) Exhaust air And: Concepts of air delivery to production areas: Recirculation systems Full fresh-air systems In this session we want you to achieve the following: 1. To understand the key issues in relation to quality assurance and quality control. 2. To understand the special needs in terms of organization, procedures, processes and resources, including staffing. 3. In your group session, to look at the special situations and problems that you face in these areas in your country, and to develop practical solutions. This area of work is potentially difficult because of the need to understand clearly the difference between quality management, quality assurance (QA) and quality control (QC). We will be looking at the issues that can arise in implementation, and at your own experiences. We will consider questions such as what to do about the owner of a factory who insists on appointing his/her son or daughter, who is not qualified, into a position of responsibility, and what to do about a factory that insists that it can manufacture penicillin products without cross-contamination risk, in the same equipment and premises used for manufacture of other types of product.
23 World Health Organization
31 March, 2017 HVAC + Production Room Exhaust air Return air (recirculated) Fresh air (make-up air) Supply air Air types There are different air types to be considered within the air handling system: Fresh air (if the plant is of the re-circulation type, it is necessary to replace some of the re-circulating air with fresh air, which is then called make-up air). A proportion of about 15% fresh air is normal, but this proportion can vary, depending on factors such as number of people, National Regulatory Authority requirements, the presence of certain substances in the air, leakage due to pressure control, etc. Supply air to the rooms Exhaust air from the rooms Return air (about 85% is being re-circulated)
24 World Health Organization
HVAC 31 March, 2017 Recirculation systems There should be no risk of contamination and cross- contamination when air is recirculated Normally, HEPA filters (EN1822 H13) needed in supply air stream Not required in single product facility with no risk of cross-contamination Not required where no dust generation (e.g. secondary packaging) HEPA filters placed in AHU or terminally Dust from highly toxic processes should not be recirculated 7.2 Recirculation system 7.2.1 There should be no risk of contamination or cross-contamination (including by fumes and volatiles) due to recirculation of air. 7.2.2 Depending on the airborne contaminants in the return-air system it may be acceptable to use recirculated air, provided that HEPA fi lters are installed in the supply air stream to remove contaminants and thus prevent cross-contamination. The HEPA fi lters for this application should have an EN1822 classifi cation of H13. 7.2.3 HEPA fi lters may not be required where the air-handling system is serving a single product facility and there is evidence that cross-contamination would not be possible. 7.2.4 Recirculation of air from areas where pharmaceutical dust is not generated such as secondary packing, may not require HEPA fi lters in the system. 7.2.5 HEPA fi lters may be located in the air-handling unit or placed terminally. 7.2.6 Air containing dust from highly toxic processes should never be recirculated to the HVAC system. 7.2.1 – 7.2.6
25 World Health Organization
31 March, 2017 HVAC Ventilation with recirculated air + make-up air Exhaust Unit This slide illustrates a typical re-circulated air setup, where a central unit distributes a mixture of fresh and re-circulated air to different production rooms. A part of the exhaust air is collected in a central duct, treated (filtered) and exhausted. The rest is re-circulated (dotted line). With control dampers, the proportions of fresh and re-circulated air can be adjusted. Central Air-Handling Unit Return air
26 World Health Organization
HVAC World Health Organization 31 March, 2017
28 World Health Organization
31 March, 2017 HVAC Ventilation with 100% fresh air (no air recirculation) Washer (optional) Exhaust Unit W This slide illustrates a typical 100% fresh air setup, where a central unit distributes the fresh, treated air to different production rooms. The exhaust air is collected in a central duct, treated (filtered or washed) and eliminated. The degree of exhaust air filtration will depend on contaminants in the exhaust air and also on environmental regulations. Central Air-Handling Unit Production Rooms
29 World Health Organization
HVAC World Health Organization 31 March, 2017
World Health Organization
Published byMorgan Porter Modified over 7 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
22 April, 2017 Supplementary Training Modules on Good Manufacturing Practice Heating, Ventilation and Air- Conditioning (HVAC) Part 3: HVAC systems and components WHO Technical Report Series, No. 961, Annex 5 Part 3: HVAC systems and components Section 7
2 World Health Organization
22 April, 2017 HVAC Objectives In the following slides, we will study the components of air- handling systems in order to: Become familiar with the components Know their functions Become aware of possible problems Objectives: For you, as inspectors, to be able to judge whether the air handling systems which you encounter during your factory inspections are adequate or not, it is necessary to know how such systems work, and to be aware of what problems may arise in terms of the components of the system. Therefore, the objectives of this part of module 3 are to study the components of air handling systems in order to: Become familiar with the components Know their functions Become aware of possible problems
3 World Health Organization
22 April, 2017 HVAC Main subsystems + Production Room Exhaust air treatment Central air handling unit Terminal air treatment at production room level Fresh air treatment (make-up air) To understand the air handling systems, it is necessary to know what their components are. A conventional Air Handling System has 4 sub-systems: 1. Air handling of the incoming (fresh) air: elimination of coarse contaminants and protection from frost if necessary. In the case of air re-circulation, the fresh air is also called make-up air. 2. Central air handling unit (AHU), where the air will be conditioned (heated, cooled, humidified or de-humidified and filtered), and where fresh air and re-circulated air, if any, (indicated here by the dotted line) will be mixed. 3. Air handling in the rooms under consideration (pressure differential system, additional filtration, air distribution). 4. Air exhaust system (filtration).
4 World Health Organization
HVAC 22 April, 2017 Components Components in HVAC may include, depending on need: Filters Fans no fan failure; including supply air fans, return air fans, exhaust air fan, dust extract system fans Driers Drying of air with chemical driers, e.g. rotating desiccant wheel Frost coils for preheating air 7.1 General 7.1.1 There should be no failure of a supply air fan, return air fan, exhaust air fan or dust extract system fan. Failure can cause a system imbalance, resulting in a pressure cascade malfunction with a resultant airfl ow reversal. 7.1.2 A schematic diagram of the airfl ow for a typical system serving a low humidity suite is represented in Fig. 23. 7.1.3 Air should be dried with a chemical drier (e.g. a rotating desiccant wheel which is continuously regenerated by means of passing hot air through one segment of the wheel). 7.1.4 The fi gure illustrates the chemical drier handling part of the fresh air/return air mixture on a by-pass fl ow. The location of the chemical drier should be considered in the design phase. Examples of appropriate locations include: — full fl ow of fresh/return air; — partial handling of fresh/return air (by-pass airfl ow); — return air only; — fresh air only; or — pre-cooled air with any of the above alternatives. 7.1.5 Possible additional components that may be required should be considered depending on the climatic conditions and locations. These may include items such as: — frost coils on fresh air inlets in very cold climates to preheat the air; — snow eliminators to prevent snow entering air inlets and blocking airfl ow; — dust eliminators on air inlets in arid and dusty locations; — moisture eliminators in humid areas with high rainfall; and — fresh air pre-cooling coils for very hot or humid climates. 7.1.6 Appropriate alarm systems should be in place to alert personnel if a critical fan fails. 7.1.7 Low-level return or exhaust air grilles are usually preferred. However, where this is not possible, a higher air change rate may be needed to achieve a specifi ed clean area classifi cation, e.g. where ceiling return air grilles are used. 7.1.1 – 7.1.6
5 World Health Organization
HVAC 22 April, 2017 General Pharmaceutical products should be manufactured in areas of appropriate cleanliness Prevent contamination and cross-contamination Design of HVAC dependent on various factors e.g. Outside air quality Recirculation of air (or not) Products and range of products Risk assessment to determine clean room conditions. 7. 1. General 7.1.1 The required degree of air cleanliness in most OSD manufacturing facilities can normally be achieved without the use of high-efficiency particulate air (HEPA) filters, provided the air is not re-circulated or in the case of a single-product facility. Many open product zones of OSD form facilities are capable of meeting ISO Class 8 or Grade D, “at-rest” condition, measured against particle sizes of 0.5 ìm and 5 ìm, but cleanliness may not necessarily be classified as such by manufacturers. A risk assessment should be carried out to determine the cleanroom conditions required and the extent of validation required. 7.1.1
6 World Health Organization
HVAC 22 April, 2017 General Two basic concepts of air delivery a re-circulation system, and a full fresh air system (100% outside air supply). Recirculation – determine the amount of fresh air based on criteria: to compensate for leakage and loss to comply with national building regulations; and for odour control. 7.1.2 There are two basic concepts of air delivery to pharmaceutical production facilities: a re-circulation system, and a full fresh air system (100% outside air supply). For recirculation systems the amount of fresh air should not be determined arbitrarily on a percentage basis, but, for example, by the following criteria: sufficient fresh air to compensate for leakage from the facility and loss through exhaust air systems; sufficient fresh air to comply with national building regulations; and sufficient fresh air for odour control. 7.1.2
8 World Health Organization
HVAC 22 April, 2017 Air distribution Positioning of supply and extract grilles to provide effective room flushing. Low-level return or exhaust air grilles preferred. If not possible, a higher air change rate may be needed to achieve a specified clean area condition, e.g. where ceiling return air grilles are used. There may be alternative locations for return air 7.2. Air distribution 7.2.1 The positioning of supply and extract grilles should be such as to provide effective room flushing. Low-level return or exhaust air grilles are usually preferred. However, where this is not possible, a higher air change rate may be needed to achieve a specified clean area condition, e.g. where ceiling return air grilles are used. 7.2.2 There may be alternative locations for return air. For example, referring to the Figure Room 1 (low-level return air) and Room 2 (ceiling return air). The airflow diagram in the Figure is an example of a typical system with a lower clean area condition. 7.2.1 – 7.2.2
9 World Health Organization
HVAC World Health Organization 22 April, 2017 The airflow schematics of the system indicates air-handling units with return air or recirculated air, having a percentage of fresh air added. Depending on product characteristics and dust loading it is sometimes preferable to fit filters on return air outlets or in return air ducting.
10 World Health Organization
22 April, 2017 This figure is a schematic diagram of an air-handling system serving rooms with horizontal unidirectional flow, vertical unidirectional flow and turbulent flow, for rooms 3, 4 and 5, respectively.
11 World Health Organization
22 April, 2017 HVAC + Production Room Exhaust air Return air (recirculated) Fresh air (make-up air) Supply air Air types There are different air types to be considered within the air handling system: Fresh air (if the plant is of the re-circulation type, it is necessary to replace some of the re-circulating air with fresh air, which is then called make-up air). A proportion of about 15% fresh air is normal, but this proportion can vary, depending on factors such as number of people, National Regulatory Authority requirements, the presence of certain substances in the air, leakage due to pressure control, etc. Supply air to the rooms Exhaust air from the rooms Return air (about 85% is being re-circulated)
13 World Health Organization
22 April, 2017 HVAC Ventilation with recirculated air + make-up air Exhaust Unit This slide illustrates a typical re-circulated air setup, where a central unit distributes a mixture of fresh and re-circulated air to different production rooms. A part of the exhaust air is collected in a central duct, treated (filtered) and exhausted. The rest is re-circulated (dotted line). With control dampers, the proportions of fresh and re-circulated air can be adjusted. Central Air-Handling Unit Return air
14 World Health Organization
HVAC 22 April, 2017 Full fresh-air systems 100% fresh air normally used in a facility dealing with toxic products or solvents, where recirculation of air with contaminants should be avoided Degree of filtration of the exhaust air depends on the exhaust air contaminants and local environmental regulations HEPA filters in the exhaust system normally when handling hazardous materials 7.4. Full fresh-air systems A system operating on 100% fresh air and would normally be used in a facility dealing with toxic products or solvents, where recirculation of air with contaminants should be avoided. 7.4.1 The required degree of filtration of the exhaust air depends on the exhaust air contaminants and local environmental regulations. HEPA filters in the exhaust system would normally only be required when handling hazardous materials. 7.4
15 World Health Organization
HVAC World Health Organization 22 April, 2017 Example of air treatment in a full fresh-air system
16 World Health Organization
22 April, 2017 HVAC Ventilation with 100% fresh air (no air recirculation) Washer (optional) Exhaust Unit W This slide illustrates a typical 100% fresh air setup, where a central unit distributes the fresh, treated air to different production rooms. The exhaust air is collected in a central duct, treated (filtered or washed) and eliminated. The degree of exhaust air filtration will depend on contaminants in the exhaust air and also on environmental regulations. Central Air-Handling Unit Production Rooms
17 World Health Organization
HVAC 22 April, 2017 Energy-recovery wheels Risk assessment to determine cross-contamination risks Should not become a source of possible contamination Alternatives include crossover plate heat exchangers and water- coil heat exchangers Prevent air leakage between the supply air and exhaust air exhaust air system operates at a lower pressure than the supply system. 7.4.2 Energy-recovery wheels if used in multiproduct facilities should have been subjected to a risk assessment to determine if there is any risk of cross-contamination. When such wheels are used they should not become a source of possible contamination (see Figure 25). Note: Alternatives to the energy-recovery wheels, such as crossover plate heat exchangers and water-coil heat exchangers, may be used in multiproduct facilities. 7.4.3 The potential for air leakage between the supply air and exhaust air as it passes through the wheel should be prevented. The relative pressures between supply and exhaust air systems should be such that the exhaust air system operates at a lower pressure than the supply system. 7.4.2 –
18 World Health Organization
HVAC World Health Organization 22 April, 2017 Energy-recovery wheels
19 World Health Organization
HVAC World Health Organization 22 April, 2017 The schematic diagram of the airflow for a typical system serving a low relative humidity suite is represented here. Air can be dried with a chemical drier (e.g. a rotating desiccant wheel which is continuously regenerated by means of passing hot air through one segment of the wheel). Alternative methods of drying air are also available. The figure illustrates the chemical drier handling part of the fresh air/return air mixture on a bypass flow. The location of the chemical drier should be considered in the design phase. The practice of locating the complete chemical drier unit in the production cubicle is not recommended as this could be a source of contamination or cross-contamination. Examples of appropriate locations for the drying wheel could include: — full flow of fresh/return air; — partial handling of fresh/return air (bypass airflow); — return air only; — fresh air only; or — pre-cooled air with any of the above alternatives.
20 World Health Organization
HVAC 22 April, 2017 Components Components in HVAC may include, depending on need: frost coils to preheat the air reheaters for humidity control, moisture eliminators automatic air volume control devices sound attenuators snow eliminators, dust eliminators, fresh air precooling coils 7.5.3 Possible additional components that may be required in air handling should be considered depending on the climatic conditions and locations. These may include items such as: — frost coils on fresh air inlets in very cold climates to preheat the air; — reheaters for humidity control — automatic air volume control devices — sound attenuators — snow eliminators to prevent snow entering air inlets and blocking airflow; — dust eliminators on air inlets in arid and dusty locations; — moisture eliminators in humid areas with high rainfall; and — fresh air precooling coils for very hot or humid climates. 7.5.3
21 World Health Organization
22 April, 2017 HVAC Filter Silencer Terminal filter Weather louvre Control damper Fan Flow rate controller Humidifier Heating coil Cooling coil with droplet separator Production Room Overview components + Prefilter Exhaust Air Grille Heater Another way to look at an air handling system is to consider the different components and to know their function. Some of the components, particularly the filters, are essential to ensure the quality of the air. We will later consider individual components in detail. Of course, a well-designed air handling system must not only be properly designed, but also properly installed, qualified and maintained (sealed ducts, tight filters). (The trainer should make the audience aware that this slide is just an example, and that all components may not necessarily be present in each system.) Secondary Filter Recirculated air
22 World Health Organization
22 April, 2017 HVAC Components (1) Weather louvre Silencer Flow rate controller Control damper To prevent insects, leaves, dirt and rain from entering To reduce noise caused by air circulation Automated adjustment of volume of air (night and day, pressure control) Fixed adjustment of volume of air A typical HVAC unit consists of a small number of elements only. It is important that these elements are compatible, properly installed, and fulfilling their goal. Whereas a weather louvre and silencer are less critical elements, the components associated with the flow rate control are essential, as they allow adjustment of the air volumes supplied to the rooms, which in turn forms the base for a pressure differential concept: to have an automated or a fixed system is largely a financial matter, but a fixed system is more difficult to set up. Silencer – check internal lining material of silencer as this can cause contamination.
23 World Health Organization
22 April, 2017 HVAC Components (2) Heating unit Cooling unit/ dehumidifier Humidifier Filters Ducts To heat the air to the proper temperature To cool the air to the required temperature or to remove moisture from the air To bring the air to the proper humidity, if too low To eliminate particles of predetermined dimensions and/or microorganisms To transport the air Heating and cooling units (batteries), as well as humidifiers are used to adjust the climate in the room (temperature and humidity). Special de-humidifiers, on a dessiccant base, will be addressed later. Filters are one of the main components, as they determine the size of airborne particles that pass through them, and thus the hygiene class. It is wise to protect the finer filters by pre-filters, thus extending their life cycles, and making them less prone to clogging. Ducts transport the air from the air handling units to and from the rooms. Inspectors must verify that ducts do not have internal insulation as this is a great source of contamination.
24 Control damper for airflow
World Health Organization 22 April, 2017 HVAC Air-handling unit Control damper for airflow Humid room air Air heater Regeneration air Adsorber wheel Dry air Dampers to control pressure differentials are important. They can be automated or fixed. As filters get dirty the system pressure losses increase, and if airflow is not regulated, the flow decreases and pressure differentials change. This could cause flow reversal and cross-contamination. Variable speed drives for fan motors are also commonly used to control airflow. In some cases, it is necessary to have very dry air for galenical reasons in certain rooms (production of effervescent tablets and humidity sensitive products in general). To generate dry air, the air supplied to the production is passed over an adsorbant (silicagel, lithium chloride, etc.) where the humidity is removed from the air. The adsorbant is then re-generated, on a continuous or on a batch-wise base. AHU with fan Variable Speed Controller Filter Pressure Gauges De-humidification
25 Humidifier Silencer Heating and cooling units
World Health Organization 22 April, 2017 HVAC Humidifier Silencer Heating and cooling units This slide shows additional elements of the air handling units. For humidification purposes, especially in clean areas, high purity water should be used, to avoid contamination. The silencer is not important from a GMP point of view, but from an environmental one, as ventilation units can be very noisy. Be sure that the silencers are manufactured of suitable materials as the linings of standard silencers can contaminate air with particulates. Depending on the local legislation, the installation of silencers can be mandatory. The cooling unit is important during the hot season. Be aware that stagnating water (condensed water) can bring bacterial growth, which can contaminate the filters, pass through them (depending on their retention properties) and end up contaminating production areas. It is essential that there is no stagnating water. Cooling coils can be sanitized as well. Do remember that, if filters are not properly maintained, micro-organisms may grow through the filters and be carried towards the production rooms.
26 World Health Organization
22 April, 2017 HVAC HEPA or tertiary filter Primary panel filter This slide shows Primary Panel filters, which are used mainly for lower filtration efficiency or as pre-filters Secondary filters, consisting of mini-pleated media or filter bags, and is used for higher filtration efficiency. HEPA or tertiary filters, usually being the final filter in the system, providing the highest filtration efficiency. Though there is a strong relationship between filter efficiency and cleanroom class, a filter of a high efficiency does not guarantee a high cleanroom class, as many other elements play a role, such as Air flow (how the air is extracted, how well the room is “flushed”) Air speed and number of air changes Positions of air terminals Layout and presence of objects Personnel and clothing Equipment (not all machines are designed to operate in a clean environment!) Proper installation and proper maintenance Secondary filter
27 Swirl Type air diffusors with terminal filters
World Health Organization 22 April, 2017 HVAC 1 2 3 4 The air flows into the rooms via so-called registers (diffusors), which are built and installed in such a way that the air is distributed evenly. Machinery or furniture can block the passage of air from the register to the exhaust point, creating unflushed zones, where counts of particles and micro-organisms could be higher. It is therefore important to consider the content of a clean room, when planning the HVAC system. In many cases, the terminal filter panel and diffusors are incorporated into one unit. It is also important that the air diffuser supplies air evenly and does not induce the circulation of dust in the room – as illustrated by the next slide. Swirl Type air diffusors with terminal filters 1 Filter 2 Tightening frame 3 Register outlet 4 Screw fixation for register
Презентация на тему World Health Organization (WHO)
Слайды и текст этой презентации
World Health Organization (WHO)
Presented By
Hari Prasad Kafle
I D # 07MPH003
FHMS; AAIDU
World Health Organization is established in 7th April 1948.
It is a specialized, non-political, health agency of United Nation with headquarter of Geneva, Switzerland.
It is responsible for providing leadership on global health matters.
Every year 7th April, is celebrated as “World Health Day”
“The attainment by all people the highest level of health”
“To lead strategic collaborative efforts among Member States and other partners to promote equity in health, to combat disease, and to improve the quality of, and lengthen, the lives of the all peoples of the world.”
Member Countries (193)
World Health Assembly
It is the Supreme governing body of the organization.
It meets annually generally in the month of May and in headquarter Geneva.
Main functions of assembly are:
To determine international health policy and program
To review the work of past year.
To approve the budget.
To elect member state to designate a person to serve for 3 year on executive board.
The board composed of at least 18 members. Now there are 34 members.
At least 3 members elected from each region.
They are composed of Technically qualified persons in the field of Health.
The board meets at least twice a year.
The main function of board is to give effect to the decisions and policies of the assembly.
It has also power to take action in an emergency such as epidemics, earthquakes, floods etc.
Secretariat is Headed by the Director General who is the chief of technical and administrative officer of the organization.
There are 5 assistant Director General and there responsibility is assigned by DG in different Divisions.
WHO Secretariat is composed of 14 different divisions:
Divisions of Secretariat
Epidemiological surveillance and health situation and trend assessment
Communicable Disease
Vector biology and control
Environmental Health
Public information and education for health
Diagnostic, therapeutic and rehabilitative technology
Divisions of Secretariat
Mental health
Strengthening of health services
Family health
Non communicable disease
Health manpower development
Information system supports
Personal and general services
Budget and finance
Regions Headquarters
South East Asia New Delhi (India)
Africa Brazzaville (Congo)
American Washington DC (U.S.A.)
Europe Copenhagen (Denmark)
Eastern Mediterranean Alexandria (Egypt)
Western Pacific Manila (Philippines)
193 Member states among which 191 Members and 2 Associate members; Niue and the Cook Islands.
All UN Member states except 2 Non UN members States; Liechtenstein and Switzerland.
Main Working Areas
Prevention and control of specific disease
Development of comprehensive health services
Family health
Environmental health
Health statistics
Bio-medical researches
Health literatures and information
Cooperation with other organizations
Global Health Situation
Global Health Situation
Providing support to countries in moving to universal coverage with effective public health interventions;
Strengthening global health security;
Generating and sustaining action across sectors to modify the behavioural, social, economic and environmental determinants of health;
Increasing institutional capacities to deliver core public health functions under the strengthened governance of ministries of health;
Strengthening WHO’s leadership at global and regional levels and supporting the work of governments at country level.
Implementing the Eleventh General Programme of Work
Role in Public Health
Providing leadership on matters critical to health and engaging in partnerships where joint action is needed;
Shaping the research agenda and stimulating the generation, translation and dissemination of valuable knowledge;
Setting norms and standards and promoting and monitoring their implementation;
Role in Public Health
Articulating ethical and evidence-based policy options;
Providing technical support, catalyzing change, and building sustainable institutional capacity; and
Monitoring the health situation and assessing health trends.
World Health Organization
Published byJocelyn Stanley Modified over 7 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
19 April, 2017 Stability Principles and Case Studies: Active Pharmaceutical Ingredient (API) and Finished Pharmaceutical Product (FPP) Gabriel K. Kaddu WHO Prequalification of Medicines Programme Assessment training, Copenhagen May 2014
2 Overview Purpose of stability
Section 2.3.S.7.1(a)/ 2.3.P.8.1(a): Stress Testing Section 2.3.S.7.2/ 2.3.P.8.2: Post approval Stability commitments Storage conditions Section 2.3.S.7.3/ 2.3.P.8.3: Stability Data Stability data Other stability studies Assessment Tips
3 Purpose of stability the purpose of stability testing is to: “provide evidence of how the quality of an API or FPP varies with time under the influence of a variety of environmental factors such as temperature, humidity and light.” The stability programme also includes the study of product related factors that influence the quality of the API or FPP, for example, interaction of API with excipients, container-closure systems and packaging materials.
4 API- 2.3.S.7.1 (a): Summary of stress testing
Stress testing: can help identify the likely degradation products which, in turn, can help to establish the degradation pathways and the intrinsic stability of the molecule and validate the stability-indicating power of the analytical procedures used. Stress testing may be carried out on a single batch of the API
5 API- 2.3.S.7.1 (a): Summary of stress testing
The objective of stress testing is not to completely degrade the API but to cause degradation to occur to a small extent, typically 10–30% loss of API by assay when compared with non-degraded API. This target is chosen so that some degradation occurs, but not enough to generate secondary products. For this reason the conditions and duration may need to be varied when the API is especially susceptible to a particular stress factor. In the total absence of degradation products after 10 days the API is considered stable under the particular stress condition.
6 API- 2.3.S.7.1 (a): Summary of stress testing
Stress conditions Treatment Results Heat Humidity Oxidation Photolysis Acid Base Other
7 FPP- Section 2.3.P.8.1(a): Stress testing
Formal stress testing for the FPP is not required, except: Light stressing should be shown for the FPP (ICH Q1B) If the API was not demonstrated to be light stable, and If the packaging has not been demonstrated to be light protective Stress testing is required as part of method validation, to show the assay and/or related compounds method is stability-indicating.
8 Generic Guideline “The quality guideline (TRS 970 Annex 4, Section P.8.1) states, “If “protect from light” is stated in one of the officially recognized pharmacopoeias for the API or FPP, it is sufficient to state “protect from light” on labelling, in lieu of photostability studies when the container-closure system is shown to be light protective.”
9 Light stress testing or photo-protective packaging
We can look at: “Protect from light” warnings in monographs “Protect from light” in APIMF Photo stress testing Packaging
10 FPP photostability testing should be conducted on at least one primary batch of the FPP if appropriate. If “protect from light” is stated in one of the officially recognized pharmacopoeias for the API or FPP it is sufficient to state “protect from light” on labelling, in lieu of photostability studies, when the container-closure system is shown to be light protective.
11 Light stressing/Light transmission
What is needed: Note that photostability testing according to ICH Q1B is “required”, however we use a pragmatic approach. Either photostability demonstration (progression of API, FPP exposed, FPP in package) or light transmission data. photostability testing should be conducted on at least one primary batch of the FPP if appropriate.
12 Conclusion: Light stressing
Product should be shown to be light stable by photostability studies showing the dosage form or dosage form in packaging is photostable. By light transmission studies showing adequate protection of the packaging. for blisters, which are not light protective, store blisters in carton (must be clear in QIS and on labels) If product is not light stable; label should state that protect from light and container should be light protective – light transmission tests should be carried out on the container closure system
13 Storage Conditions Climatic Zones Zone I: 21ºC/45% RH
Zone II: 25ºC/60% RH (subtropical) Zone III: 30ºC/35% RH(hot/ dry) Zone IVA: 30ºC/65% RH (hot/ humid) Zone IVB: 30ºC/75% RH (hot/very humid) Long term stability studies should cover all climatic zones if possible
14 3.2.S.7.1(b)/ 3.2.P.8.1(b): Accelerated and Long term testing
Stability data must demonstrate stability of the medicinal product throughout its intended shelf-life under the climatic conditions prevalent in the target countries. Effective as of September 2011, the required long-term storage conditions for the WHO Prequalification of Medicines Programme are 30ºC ± 2º C/75% ± 5% RH. The use of alternative long-term conditions should be justified and should be supported with appropriate evidence.
15 When the API or FPP stability studies were not conducted at PQ conditions:
The required long-term stability condition within the WHO Prequalification programme is 30ºC/75%RH for both API and FPP (Note: 30±2°C/ 65±5% RH is also acceptable for API), unless documented evidence can be provided that storage under these conditions is not possible due to the inherent chemical instability of the API. In light of this, please provide long-term stability data for your API at 30C/75%RH or provide documented evidence that such storage conditions are inappropriate.
16 When the API or FPP stability studies were not conducted at PQ conditions: API
If long-term stability data at 30±2°C/ 65±5% RH or 30ºC/75%RH is not available for API, please commit to generating such data on the next three production scale batches of XXXXXXXX; provide a signed detailed protocol describing this stability programme; and commit to providing an amendment to alter the recommended storage conditions and retest period once 24 months stability data has been generated.
17 FPP In cases where the delay in accepting the dossier in order to obtain the stability data at 30ºC/75%RH is unacceptable: The required long-term stability condition within the WHO Prequalification programme is 30ºC/75%RH, unless documented evidence can be provided that storage under these conditions is not possible due to the inherent instability of the product. In light of this, please provide long-term stability data for your product at 30C/75%RH or provide documented evidence that such storage conditions are inappropriate.
18 FPP If long-term stability data at 30ºC/75%RH is not available, you are required to commit to initiating studies at 30ºC/75%RH on the required number of primary batches; provide a signed detailed protocol describing the stability programme including a commitment for the date of initiation of studies, no more than 60 days from today’s date.
20 3.2.S.7.1(b)/ 3.2.P.8.1(b): Accelerated and Long term testing
Condition Storage temperature (ºC) Relative humidity (%) Minimum time period (months) Accelerated 40 ± 2 75 ± 5 6 Long-term 30 ± 2 (for both API and FPP) 65 ± 5 (for API)
21 Exceptions: Reproductive Health and 2nd line TB drugs 1
the requirement for stability at the time of submission only, may be reduced to; no less than 3 months accelerated, plus 3 months long-term data, for not less than two primary batches of at least pilot scale or in the case of an uncomplicated1 FPP (e.g. immediate-release solid FPPs (with noted exceptions), non-sterile solutions), not less than one batch of at least pilot scale and a second batch which may be smaller (e.g. for solid oral dosage forms, or tablets or capsules) of each proposed strength of the FPP.
22 Exceptions: Reproductive Health and 2nd line TB drugs 2
One of the primary batches (also called exhibit, pre-approval, registration, submission, or test batches) should be the batch that was used for bioequivalence studies. All prequalification requirements for final acceptability of the dossier remain in effect, without exception; the submission of updated stability data during the assessment period as it becomes available, and a commitment to provide: stability data on three production batches, and process validation of three consecutive production batches, to be completed prior to marketing.
23 3.2.S.7.1(b)/ 3.2.P.8.1(b): Accelerated and Long term testing
Other storage conditions are outlined in the WHO stability guidelines: for FPPs packaged in impermeable and semi-permeable containers and those intended for storage in a refrigerator and in a freezer. FPPs intended for storage below −20°C should be treated on a case-by-case basis. Weight loss from plastic containers should be reported over the shelf-life (Liquids only).
24 Stability of aqueous liquids
Aqueous Liquids in semi-permeable containers – ICH Q1 and TRS953: long term studies should be at 25°C/40%RH or (for PQ) 30°C/35%RH plus 40°C/25%RH Q1A: “Aqueous-based products packaged in semi-permeable containers should be evaluated for potential water loss in addition to physical, chemical, biological, and microbiological stability…Ultimately, it should be demonstrated that aqueous-based drug products stored in semi-permeable containers can withstand low relative humidity environments. ”
25 Container closure systems 1
Semi-permeable (ICH and TRS 953): Containers that allow the passage of solvent, usually water, while preventing solute loss […] Examples of semi-permeable containers include plastic bags and semi-rigid, low-density polyethylene (LDPE) pouches for large volume parenterals (LVPs), and LDPE ampoules, bottles, and vials. HDPE bottles are considered semi-permeable for storing liquids.
26 Container closure systems 2
Impermeable Containers that provide a permanent barrier to the passage of gases or solvents, e.g. sealed aluminium tubes for semisolids, sealed glass ampoules for solutions (ICH/TRS 953) and (TRS 953 only) aluminium/aluminium blisters for solid dosage forms. (Be careful of Al/Al, it depends on the sealing.)
27 Container closure systems 3
TRS 953: “Sensitivity to moisture or potential for solvent loss is not a concern for FPPs (for liquids) packaged in impermeable containers that provide a permanent barrier to passage of moisture or solvent. Thus stability studies for products stored in impermeable containers can be conducted under any controlled or ambient relative humidity condition. Note: This does not apply to AL/ Al blisters. For Al-Al blisters, humidity in stability studies is just as important as for bottles. It is just that moisture permeability does not have to be tested for the package.
28 Section 3.2.S.7.2/ 3.2.P.8.2 Post-approval stability commitment
2.3.P.8.2(a): Primary stability study commitment 2.3.P.8.2(b): Commitment stability studies 2.3.P.8.2(c): Ongoing stability studies Parameter Details Storage conditions(s) (ºC, %RH) Batch number(s)/ Batch Size(s) Tests and acceptance criteria: Description Assay etc Testing frequency Container closure system
29 Section 3.2.S.7.2/ 3.2.P.8.2: Post-approval stability protocol
number of batch(es) and different batch sizes, if applicable; relevant physical, chemical, microbiological and biological test methods; acceptance criteria; reference to test methods; description of the container-closure system(s); testing frequency; description of the conditions of storage
30 Information on stability studies
– storage conditions; – strength; – batch number, including the API batch number(s) and manufacturer(s); – batch size; – completed (and proposed) test intervals. – container-closure system including orientation (e.g. erect, inverted, on-side) where applicable; important for liquids and semi-solids (e.g. erect, inverted, on-side): This is to study the possible interaction of the container closure system (the container and the closure) with the product.
31 Section 3.2.S.7.3/ 3.2.P.8.3: Stability data
Results of the stability studies should be presented in an appropriate format : tabular, graphical, Narrative Summary of analytical methods Information on the analytical procedures used to generate the data and validation of these procedures should be included.
33 API: Retest period A retest period should be derived from the stability information and should be displayed on the container label. After this retest period a batch of API destined for use in the manufacture of an FPP could be retested and then, if in compliance with the specification, could be used immediately (e.g. within 30 days). If retested and found compliant, the batch does not receive an additional period corresponding to the time established for the retest period. However, an API batch can be retested multiple times and a different portion of the batch used after each retest, as long as it continues to comply with the specification. For APIs known to be labile (e.g. certain antibiotics) it is more appropriate to establish a shelf-life than a retest period.
34 Change in specifications
A change in specifications is a stability issue.
35 Stability issue – specifications have changed
Often during assessment specs are revised (by request usually) but there is no attention paid to whether stability data supports the change. [This is another reason why it is so important to compare previous specs to the revised version provided.] It is not sufficient to accept only a commitment for results for the new specification. When we accept a shelf-life we are saying we are satisfied that the specifications will be met based on data we have assessed.
36 Stability issue – specifications have changed
If the revised limits can be assessed using current data, just ensure the new limits are met by most recent stability data. Example tightened impurity limits: we can usually tell whether the new limits have been met by reviewing the available data Imp A: up to 0.2% reported; meets new limit of NMT 0.1% Exception in this case is TLC: semi-quantitative results were provided: Imp A:
37 Stability issue – specifications have changed
The revised limits may require new testing/reporting, such as inclusion of a limit for a new impurity or a different dissolution timepoint. In these cases, the available data is insufficient: 1. If studies are completed: Request available data on retained samples if not far beyond expiry, or available new studies 2. If studies are ongoing: Request that data at subsequent stations include the revised limits.
38 Stability-indicating parameters
Stability-indicating parameters must be understood for the API and FPP. API: any parameter susceptible to change (appearance, assay, impurities*, others specific to the API e.g. polymorphism if necessary) Why all specified impurities, and not just degradation products for the API? The way to determine if an impurity is also a degradant is to do the stability and monitor known impurities. A process impurity could also turn out to be a degradant. The degradants for an API by one manufacturing process may differ from that of another, for example due to the presence of trace metals or other reagents.
39 Stability-indicating parameters
FPP: Physical (description, moisture, quality parameters (DT, dissolution)); Moisture is particularly important for solid orals in blisters and strips, and moisture-sensitive products in bottles. Chemical (assay, degradants, preservatives); Efficacy of additives Container/closure interactions, when applicable (e.g. liquid in plastic);
40 Evaluation and Extrapolation of results
if significant change was not observed within 6 months at accelerated condition and the data show little or no variability, the proposed shelf-life could be up to twice the period covered by the long-term data, but should not exceed the long-term data by more than 12 months The proposed storage statement and shelf-life (and in-use storage conditions and in-use period, if applicable) for the FPP should be provided. The recommended labelling statements for use based on the stability studies, are provided in the WHO stability guidelines
41 Provisional shelf-lives 1
Ideally, these would not exist. As long as there are significant question(s) and the data did not cover the proposed shelf-life, updated data should be requested/provided. Example problem: 12 mo data provided in blisters and then the study was discontinued. A provisional 24 mo shelf-life was accepted. Is this okay? No. You can’t extrapolate based on data for batches when the study on these batches will not be continued over the extrapolated period.
42 Provisional shelf-lives 2
Never accept extrapolation for cancelled studies. ICH Q1E: “a retest period or shelf life granted on the basis of extrapolation should always be verified by additional long-term stability data as soon as these data become available.” WHY? 1) we don’t know why the studies were cancelled. 2) we don’t have the normal assurance of the “primary stability commitment”, and 3) if they cancelled studies (in the above case they claim because of low market potential), the odds of them starting a new study are slim. This is very risky and against all practices.
43 Updated stability data and extrapolation – what NOT to say
“Please provide update stability data. In order to support of 24 months shelf life of the product, long term stability data up to at least 12 months should be provided, with commitment to continue long term testing for a period of time sufficient to cover the whole proposed shelf life.” The above suggests that we will accept 12 months data, and extrapolate on this, even if longer data is available. What TO say: “In order to support the proposed shelf-life, you are requested to provide available updated stability data.”
44 Extrapolation Ask for the available updated stability data each time comments are going out. Don’t imply that extrapolation is a guarantee as in the example question. It depends on the actual data provided. Don’t offer extrapolation unless it has specifically been proposed by the applicant.
45 2.3.S.7.1(b)/ 2.3.P.8.1(b): Summary of accelerated and long term testing
Summary of stability results observed Storage conditions (ºC, % RH) Strength and Batch number Batch size Container closure system Completed (and proposed) test intervals Test Results Description Moisture Impurities Assay
47 Hold time Studies 1 Summary of acceptable hold times
A maximum processing time of one month (30 days) is acceptable without validation. (industry standard) Any hold time for an intermediate or the bulk product >30 days must be supported by stability studies. In addition, cumulative hold times should not exceed 90 days, or additional supporting data is required. A maximum processing time of one month (30 days) is acceptable without validation. (industry standard) Maximum processing from dispensing to the final coating Any hold time for an intermediate or the bulk product >30 days must be supported by stability studies. In addition, cumulative hold times should not exceed 90 days, or additional supporting data is required. Example from previous QA talk: 16.5 months for a CR tablet (shelf-life 24 months) Blend days Compressed tablets 90 days Seal coated tablets 7 days CR coated tablets 90 days Laser drilled tablets 90 days Bulk tablets 6 months
48 Hold time studies 2 These are a type of stability study to determine hold times for example for blend, cores and final coated tablets in bulk. Necessary when hold time > 30 days or there are multiple hold times (e.g ). All stability-indicating parameters should be included in the studies. The applied limits are important. Release limits should be applied to final tablets, not just shelf-life.
49 Hold time studies 3 Beware of even short hold times for liquid intermediates. Example comment: The API-loaded suspension is allowed to be stored for three days before being further used for processing. The suspension contains HPMC and lactose, both of which could promote microbial growth. Please provide the supporting data for microbial stability of the suspension and the chemical stability of the API for the proposed waiting period of three days during processing.
50 Hold time studies 4 Common issue: a number of intermediates have hold times Beware of cumulative hold times, even if separately validated The date of production of a batch is defined as the date that the first step is performed involving combining the API with other excipients. Even if appropriate hold studies have been provided to support hold times, the hold times still may be unacceptable.
51 Hold time studies 5 Recent example (controlled release (CR) tablet):
Blend days Compressed tablets 30 days Seal coated tablets 5 days CR coated tablets 30 days Laser drilled tablets 30 days Bulk tablets months Total hold time > 5 months before packaging! Shelf-life is only 24 months. This should be questioned! E.g. Flag this if >3 months total
52 Hold time studies 6 Comment out:
You have indicated cumulative hold times prior to the stage of packaging of the product that add to over 5 months. You are reminded that the start of shelf-life is defined as the time of first mixing of an excipient with the API. You are requested to significantly reduce the hold times so that the maximum processing time will be kept at a reasonable duration (e.g. 30 days) or provide additional evidence that a product subject to the cumulative effect of the largest hold times would still meet release specifications as well as shelf-life specifications over the remaining shelf-life.
53 In-use studies 1 TRS 970 Annex 4 states: Any in-use period and associated storage conditions should be justified with experimental data, for example, after opening, reconstitution and/or dilution of any sterile and/or multidose products or after first opening of FPPs packed in bulk multidose containers (e.g. bottles of 1000s*). If applicable, the in-use period and storage conditions should be stated in the product information. *only for rifampicin containing products and highly hygroscopic products. In use period of 28 days generally accepted without data (most products).
54 In-use studies 2 Standard comment out: It is noted that a two month in-use period is claimed for the product. As outlined in the quality guideline (TRS 970 Annex 4, P.8.1 p. 184), any in-use period and associated storage conditions should be justified with experimental data. A minimum of two batches of at least pilot scale* should be subjected to the test. At least one of the batches should be chosen towards the end of its shelf-life. * May use lesser requirement for uncomplicated FPPs
55 In-use studies 3 As far as possible the test should be designed to simulate the use of the product in practice (i.e. the same bottle opened at each interval), taking into consideration the filling volume of the container. At intervals comparable to those that occur in practice, appropriate quantities should be removed by the withdrawal methods normally used and described in the product literature. In the case where the package size is inadequate for the full study: One unit (tablet or capsule) may be withdrawn and then replaced.
56 In-use studies 4 Rather than just opening the bottle, the protocol should specify that at least one capsule is picked out each time it is opened, and then it may be immediately returned to the bottle for future analysis. This is to ensure that air moves in the bottle as it is expected to during normal use.
57 Transport studies 1 It is transportation of bulk product that requires transport studies. Transportation of packaged product is not normally required (may be requested in special cases). If there are stability issues with the FPP, then study results may be requested.
58 Transport studies 2 If there are no stability issues with the FPP, we do not pursue study results. Instead a protocol is sufficient, including: at least one batch of at least pilot scale; confirmation of whether the product will be shipped in a controlled environment, with or without transportation logging devices; and If not a controlled environment, assessment of environmental conditions to which the product will be exposed during transportation, and studies to support the stability of the product in the bulk package at the extremes of the likely conditions.
59 Tips for Assessment of Stability Studies
CASE STUDY ON STABILITY STUDIES
60 Assessment Tips for stability 1
Has the applicant provided the stability protocol? Check the conditions used in the stability studies Has the applicant submitted accelerated stability studies? 40±2°C/ 75±5% RH. Has the applicant submitted long term stability studies? 30±2°C/ 75±5% RH for API and FPP (30±2°C/ 65±5% RH is also acceptable for API) except for justified cases.
61 Assessment Tips for stability 2
Check the period of the stability studies submitted: atleast 6 months for both accelerated stability studies and long term stability studies. Exceptions: RH products and 2nd line TB drugs Check the number of batches used in the stability studies Check the batch size of the batches used in the stability studies. Are they acceptable: atleast 2 batches of pilot scale or for an uncomplicated FPP, one of pilot scale and second batch which may be smaller
62 Assessment Tips for stability 3
Check the type of the container closure system including the type of the material of the cap used in the stability studies: What is the type of packaging type used including the type of cap, if applicable? Is it the same as that declared in section 2.3.P.7? Check the conditions used in the stability studies Check the period of the stability studies submitted: atleast 6 months for both accelerated stability studies and long term stability studies. Exceptions: RH products and 2nd line TB drugs
63 Assessment Tips for stability 4
Are all the stability indicating tests included? Check the shelf life specifications against the specifications used in the stability studies. Check the results for each parameter at each test station to confirm whether the results are all within the limits (specifications). No failured Is there any significant change observed in the stability study results? Summarize the trends for each parameter.
64 Assessment Tips for stability 5
Is there mass balance in the stability study results? Do the stability studies submitted support the proposed shelf life? Do the stability studies support the proposed storage condition? Does the compendia state any special storage condition for the product? The storage condition should include the provisions stated in the compendia. The container closure system should provide the necessary protection.
65 Assessment Tips for stability 6 Stability Commitments
Has the applicant submitted written and signed stability study commitments (primary stability study commitment, commitment stability study commitments and ongoing stability study commitments)? Check the batches stated under the primary stability commitment: primary batches Check the batches stated under the commitment stability studies: first three consecutive commercial batches. Check the batches stated under the ongoing stability study commitment: atleast one batch per year
66 References TRS_970- Annex 4 Generic Guideline
QA Talks by L. Paleshnuik, Lead Quality Assessor PQP. Presentation- EAC Joint Session July 2013: Assessment part XIV section P.8, L. Paleshnuik, Lead Quality Assessor PQP. Presentation- EAC Joint Session July 2013: Process of Assessment – API areas S.5, S.6, S.7, Hua Yin ICH Q1A, Q1B, Q1D, Q1E, Q2, WHO Technical Report Series, No. 953, Annex 2
67 World Health Organization
19 April, 2017 Thank you
World Health Organization
Global Tobacco Surveillance System Accomplishments and Opportunities Samira Asma Associate Director Global Tobacco Control Office on Smoking and Health. World Health Organization. 1999. ▪ We were hindered by what we didn’t know ▪ Urgency to fill the data gap was clear. World Health
World Health Organization
Presentation Transcript
Global Tobacco Surveillance SystemAccomplishments and OpportunitiesSamira AsmaAssociate DirectorGlobal Tobacco ControlOffice on Smoking and Health World Health Organization
1999 ▪We were hindered by what we didn’t know ▪ Urgency to fill the data gap was clear World Health Organization
Data Need ▪ Monitor tobacco epidemic ▪ Plan, implement and evaluate tobacco control programs World Health Organization
Global Tobacco Surveillance System: Components Youth Global Youth Tobacco Survey (GYTS) Adults Global School Personnel Survey (GSPS) Global Health Professionals Survey (GHPS)
Fundamental Principles Partnership Consistency Survey Methodology Field Procedures Data Management Techniques Sustainability Cost effective Feasibility to repeat
Opportunities • Monitor the WHO FCTC Articles • Develop, monitor and evaluate tobacco control programs • Disseminate data to expand science base
Global Youth Tobacco Survey Purpose • To enhance the capacity of countries to design, implement, and evaluate their comprehensive tobacco control programs
GYTS Topics Prevalence & consumption Knowledge & attitudes Access, availability & price Media & advertising exposure Secondhand smoke exposure Cessation School curriculum
GYTS 1999-2005 • Completed • Planned ▪Active in 164 countries ▪1.8 million students participated ▪40 countries repeating
Key GYTS Findings 1 in 7 students smoke cigarettes 1 in 4 smokers first tried by age 10 2 in 3 smokers want to quit immediately Half of students exposed to smoke in homes 80% saw tobacco ads Source: Tobacco Control 2002; 11:252-270
WHO FCTC monitoring protocol “…integrate tobacco surveillance programs into national, regional, and global health surveillance programs so that data are comparable and can be analyzed at the regional and international levels, as appropriate.”
Monitoring WHO FCTC Articles • Article 8: Protection from exposure to tobacco smoke • Article 12: Education, communication, training & public awareness • Article 13: Tobacco advertising, promotion & sponsorship • Article 14: Demand reduction measures concerning tobacco dependence & cessation • Article 16: Sales to & by minors
Percent With Tobacco Logo on Object or Offered Free Cigarettes, Global Youth Tobacco Survey by WHO Region Have Tobacco Logo on an Object Offered Free Cigarettes
Develop and Monitor Tobacco Control Programs • GYTS is being used by countries to: • Develop program • Influence policy decisions • Evaluate program effectiveness
Factors Influencing the Change in the Philippines, 2000-2004 Clean Air Act of 1999 Tobacco Regulatory Act of 2004 Extensive media coverage of Tobacco RegulatoryAct & smoke-free policy Smoking cessation programs
Global Tobacco Surveillance System: Components • Adults • Global School Personnel Survey (GSPS) • Global Health Professionals Survey (GHPS)
Purpose To collect information from school personnel concerning their tobacco use & tobacco related school policies & programs Global School Personnel Survey
GSPS Topics Tobacco use Knowledge and attitudes School policy School curriculum
Key GSPS Findings Teachers less likely to use tobacco Shortage of teaching materials Majority of teachers in favor of schools enacting policy Source: Journal of School Health 2004; 74: 3-5
Purpose To collect information from third-year students attending dental, medical, nursing, & pharmacy schools concerning their tobacco use & their tobacco related school policies & programs Global Health Professionals Survey
GHPS Topics Tobacco use Knowledge & attitudes Exposure to second-hand smoke Cessation School curriculum & training
Data Dissemination • Data Coordinating Center • Ongoing technical support • Data Release Policy • Data Bank • Data for Public Use • 153 GYTS data sets released for 85 countries • Active Data Dissemination • Dynamic Website • Publications
Global Tobacco Surveillance SystemAccomplishments and OpportunitiesSamira AsmaAssociate DirectorGlobal Tobacco ControlOffice on Smoking and Health World Health Organization
World Health Organization
Published byCory Small Modified over 7 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
Evaluation of Quality and Interchangeability of Medicinal Products 20 April, 2017 Training Workshop for Evaluators from National Medicines Regulatory Authorities in East African Community Dar Es Salaam, Tanzania Date: 10 to 14 September 2007
2 Evaluation of Quality and Interchangeability of Medicinal Products
World Health Organization 20 April, 2017 Evaluation of Quality and Interchangeability of Medicinal Products Overview of Dossier Requirements and Guidelines Presenter: Deus K. Mubangizi, pharmacist, MSc(Pharm.) Chief Inspector of Drugs, National Drug Authority WHO expert
3 Overview of Dossier Requirements and Guidelines
World Health Organization 20 April, 2017 Overview of Dossier Requirements and Guidelines Outline of presentation Objectives of presentation Structure of dossier of medicinal products, information on the CTD format Guideline on Submission of documentation for multisource FPPs Supplement 1: (dissolution testing) Supplement 2: (Extension of WHO list of stable compounds) Products registered in ICH Region and related countries Fixed-dose combinations ICH guidelines Variations
4 Overview of Dossier Requirements and Guidelines
World Health Organization 20 April, 2017 Overview of Dossier Requirements and Guidelines Objective of the presentation: To give an overview of the dossier requirements and Guidelines used or referenced during the evaluation of dossiers under the WHO Prequalification Program To demonstrate how the requirements and guidelines can be applied or used as reference during dossier evaluation
5 World Health Organization
Data in the dossier should enable us to answer the following questions: 20 April, 2017 What is the product? Is the quality presented acceptable on grounds of safety and efficacy? Is the quality presented reproducible? How long can the quality be maintained? Quality must ensure consistency of safety and efficacy during the shelf life of all batches produced.
6 Overview of Dossier Requirements and Guidelines (1)
World Health Organization 20 April, 2017 Overview of Dossier Requirements and Guidelines (1) Common Technical Document (CTD) An initiative under the ICH: Europe, Japan and USA.
7 World Health Organization
20 April, 2017 Structure of dossier of medicinal products, information on the CTD format (1) A common format for the technical documentation: significantly reduces the time and resources needed to compile applications for registration of human pharmaceuticals eases the preparation of electronic submissions Facilitates regulatory reviews and communication with the applicant by a standard document of common elements Simplifies exchange of regulatory information between Regulatory Authorities This guideline is not intended to indicate what studies are required. It merely indicates an appropriate format for the data that have been acquired.
8 World Health Organization
20 April, 2017 CTD format (2) GENERAL PRINCIPLES Text and tables should be prepared using margins that allow the document to be printed on A4 paper. The left-hand margin should be sufficiently large that information is not obscured by the method of binding. Font sizes for text and tables should be easily legible, even after photocopying. Times New Roman, 12-point font, is recommended for narrative text. Every page should be numbered. Acronyms and abbreviations should be defined the first time they are used in each module. References should be cited in accordance with the current edition of the Uniform Requirements for Manuscripts Submitted to Biomedical Journals, International Committee of Medical Journal Editors (ICMJE)1.
9 World Health Organization
20 April, 2017 CTD format (3) The CTD is organized into five modules: Module 1 is region specific. Modules 2, 3, 4, and 5 are intended to be common for all regions. Module 1. Administrative Information and Prescribing Information Should contain documents specific to each region; e.g. application forms or the proposed label for use in the region. The content and format of this module can be specified by the relevant regulatory authorities. Module 1: Administrative Information and Prescribing Information 1.1 Table of Contents of the Submission Including Module 1 1.2 Documents Specific to Each Region (for example, application forms, prescribing information)
10 World Health Organization
20 April, 2017 CTD format (4) Module 2. Common Technical Document Summaries Should begin with a general introduction to the pharmaceutical, including its pharmacological class, mode of action, and proposed clinical use. In general, the Introduction should not exceed one page. Should contain 7 sections in the following order : 2.1 Common Technical Document Table of Contents (Modules 2-5) 2.2 CTD Introduction 2.3 Quality Overall Summary 2.4 Non-clinical Overview 2.5 Clinical Overview 2.6 Non-clinical Written and Tabulated Summaries Pharmacology Pharmacokinetics Toxicology 2.7 Clinical Summary Biopharmaceutical Studies and Associated Analytical Methods Clinical Pharmacology Studies Clinical Efficacy Clinical Safety Literature References Synopses of Individual Studies
11 World Health Organization
20 April, 2017 CTD format (5) Module 3. Quality Information on Quality should be presented in the structured format described in Guideline M4Q. Module 3: Quality 3.1 Table of Contents of Module 3 3.2 Body of Data 3.3 Literature References
12 World Health Organization
20 April, 2017 CTD format (5) Module 4. Non-clinical Study Reports The non-clinical study reports should be presented in the order described in Guideline M4S. Module 4: Non-clinical Study Reports 4.1 Table of Contents of Module 4 4.2 Study Reports 4.3 Literature References
13 World Health Organization
20 April, 2017 CTD format (6) Module 5. Clinical Study Reports The human study reports and related information should be presented in the order described in Guideline M4E. Module 5: Clinical Study Reports 5.1 Table of Contents of Module 5 5.2 Tabular Listing of All Clinical Studies 5.3 Clinical Study Reports 5.4 Literature References
14 World Health Organization
20 April, 2017 CTD format: Overall Table of Contents (ToC) 1.1 ToC of Module 1 or overall ToC, including Module 1 2.1 ToC of the CTD (Mod 2,3,4,5) Module 1 Module 3 Module 4 Module 5 2.2 2.3 2.4 2.5 2.6 2.7 Module 2 3.1 ToC for Module 3 4.1 ToC for Module 4 5.1 ToC for Module 5
15 World Health Organization
20 April, 2017 CTD format: Numbering System 1.0 Regional Administrative Information 1.1 ToC of Module 1 or overall ToC, including Module 1 2.1 ToC of the CTD (Mod 2,3,4,5) 2.2 Introduction 2.3 Quality Overall Summary 2.4 Non-clinical Overview 2.5 Clinical Overview 2.7 Clinical Summary 2.6 Non-clinical Written and Tabulated Summaries Module 1 Module 3 Module 4 Module 5 2.1 2.2 2.3 2.4 2.5 2.6 2.7 1.0 Quality Nonclinical Study Reports Clinical Module 2
16 World Health Organization
20 April, 2017 CTD format: Numbering System: Module 2
17 World Health Organization
20 April, 2017 CTD format: Numbering System: Module 2
18 World Health Organization
20 April, 2017 CTD format: Numbering System: Module 3
19 World Health Organization
20 April, 2017 CTD format: Numbering System: Module 4
20 World Health Organization
20 April, 2017 CTD format: Numbering System: Module 5
21 Overview of Dossier Requirements and Guidelines (2)
World Health Organization 20 April, 2017 Overview of Dossier Requirements and Guidelines (2) Guideline on Submission of documentation for Prequalification of Multi-source (Generic) Finished Pharmaceutical Products (FPPs) Used in the Treatment of HIV/AIDs, Malaria and Tuberculosis
22 Generic Guide: Definitions
World Health Organization 20 April, 2017 Generic Guide: Definitions Active Pharmaceutical Ingredient (API) A substance or compound that is intended to be used in the manufacture of a pharmaceutical product as a therapeutically active compound (ingredient) Pharmaceutical Product Any preparation for human or veterinary use that is intended to modify or explore physiological systems or pathological states for the benefit of the recipient. Finished Pharmaceutical Product (FPP) A product that has undergone all stages of production, including packaging in its final container and labelling.
23 Multisource (Generic) product
World Health Organization 20 April, 2017 Multisource (Generic) product Multisources are Pharmaceutically equivalent (WHO definition) same amount of the same API same dosage form meet the same or comparable standards intended to be administered by the same route Multisources which are therapeutically equivalent are interchangeable
24 Quality of a Generic product
World Health Organization 20 April, 2017 Quality of a Generic product Multisource products must be of good quality and at least as safe and efficacious as existing products (WHO Manual, Blue Book, P. 29, chapter H., Interchangeability)) Equal quality with the comparator or a quality shown and assessed to be as acceptable Same Safety – Same efficacy Demonstration of pharmaceutical equivalence of the FPP including that of the API
25 World Health Organization
20 April, 2017 Generic Guide: Documentation on Quality Part to be submitted to the WHO PQ team Covering letter Product dossier on Quality part PQIF (annex 8 to the main generic guide): properly filled out in WinWord format, See mock-up PQIF on under training material and workshops, Hanoi, Vietnam, January 2006 Product dossier of Efficacy part BTIF (annex 7 to the main generic guide) properly filled out in WinWord format
27 World Health Organization
20 April, 2017 Generic Guide: Quality dossier / Section 2 Active Pharmaceutical Ingredient (API)
28 Generic Guide: Quality/Section 2: API
World Health Organization 20 April, 2017 Generic Guide: Quality/Section 2: API Scientific data on the API can be submitted in the following order of preference A valid Certificate of Suitability (CoS) or CEP, latest version, with all its annexes issued by EDQM An APIMF (Active Pharmaceutical Ingredient Master File) submitted by the API manufacturer, containing the whole information requested in section 2 Complete submission of data requested in Section 2
29 Generic Guide: Quality/Section 2: API Complete submission option
World Health Organization Generic Guide: Quality/Section 2: API Complete submission option 20 April, 2017 2.1. Nomenclature (INN, chemical name, CAS No.) 2.2. Properties of the API** 2.3. Site(s) of manufacture 2.4. Route(s) of synthesis** 2.5. Specifications** 2.6. Container- closure system 2.7. Stability testing ** The requirements may differ depending on if the API is pharmacopoeial or non-pharmacopoeial
30 Generic Guide: Quality/Section 2: API
World Health Organization 20 April, 2017 Generic Guide: Quality/Section 2: API TB drugs / 6th EoI Rifampicin (rifampin) Ph. Eur., USP, BP, Ph. Int. Ethambutol 2HCl Ph. Eur., USP, BP, Ph. Int., JP Pyrazinamide Ph. Eur., USP, BP, Ph. Int., JP Isoniazid Ph. Eur., USP, BP, Ph. Int., JP Streptomycin sulfate Ph. Eur., USP, BP, Ph. Int. Amikacin Ph. Eur., USP, BP, Ph. Int., JP Kanamycin Ph. Eur., USP, BP Capreomycin USP, Ph. Int. Cycloserine USP, JP Ethionamide Ph. Eur., USP, BP, Ph. Int., JP Ofloxacin Ph. Eur., USP, BP Prothionamide Ph. Int., JP p-Aminosalicylic acid (and sodium salt) Ph. Eur., USP
31 Generic Guide: Quality/Section 2: API
World Health Organization 20 April, 2017 Generic Guide: Quality/Section 2: API Artemisinin based antimalarial drugs / EoI May 2005 Artesunate Ph. Int. Artemether Ph. Int. Artemotil (arte-ether) Ph. Int. Amodiaquine Ph. Int. mefloquine Sulphadoxine Pyrimethamine Lumefantrine Non-pharmacopoeial
32 Generic Guide: Quality/Section 2: API
World Health Organization 20 April, 2017 Generic Guide: Quality/Section 2: API Antiretrovirals Abacavir Ph. Int. Didanosine Ph. Int. Efavirenz Ph. Int. Indinavir Ph. Int., USP Lamivudine Ph. Eur., USP, BP, Ph. Int. Nelfinavir Ph. Int. Nevirapine Ph. Int., USP, Ph. Int. Stavudine Ph. Eur., USP, BP, Ph. Int. Saquinavir Ph. Int., USP Ritonavir Ph. Int., USP Zidovudine Ph.Int., USP, Ph. Eur., BP Tenofovir and Emtricitabine Non-pharmacopoeial
33 World Health Organization
20 April, 2017 Generic Guide: Quality/Section 2: API, Certification of Suitability (CoS) / CEP Option Issued by EDQM for substances described in the Ph. Eur. 2 types of CEPs: quality CEP and TSE CEP Information which can be found on a quality CEP CEP reference, CEP holder, site of manufacture of the substance, monograph according to which the dossier is evaluated, additional impurities and residual solvents not mentioned in the monograph, additional methods to those of the monograph are appended, re-test period with packaging system and storage condition (if applicable), date of validity of the CEP A quality CEP certifies that the quality of the substance can be checked according to the Ph. Eur. by applying the analytical methods described in the Ph. Eur. monograph supplemented by those appended to the CEP.
34 Generic Guide: Quality/Section 2: API, APIMF Option
World Health Organization 20 April, 2017 Generic Guide: Quality/Section 2: API, APIMF Option Procedure implemented since January 2007, To protect the «know-how» of the manufacturer of the API While giving the whole information on manufacture of the API to the WHO PQ team of assessors While giving a part of the information to the applicant to Prequalification/ manufacturer of the finished product An APIMF is composed of: Applicant’s /Open part + Restricted / Closed part Manufacturer of the API should make available to the applicant to Prequalification the Applicant’s part + Letter of access
35 Generic Guide: Quality/Section 2: API. APIMF Option
World Health Organization 20 April, 2017 Generic Guide: Quality/Section 2: API. APIMF Option Manufacturer of the API should submit on the other hand the Applicant’s part + Restricted + Letter of access to WHO team An APIMF is to be submitted only in support of a FPP dossier An APIMF is not an independent dossier of API Scope open to pharmacopoeial and non-pharmacopoeial APIs Scope of APIMF only open to APIs ≠ US and Canadian master file procedures See annex 1 of the APIMF guide for the content of an APIMF Content of APIMF corresponds to data required in section 2 of the prequalification quality dossier without difference between pharmacopoeial and non-pharmacopoeial APIs
36 World Health Organization
20 April, 2017 Generic Guide: Quality dossier / Section 3 Finished Pharmaceutical Product (FPP)
37 Generic Guide: Quality/Section 3: FPP
World Health Organization 20 April, 2017 Generic Guide: Quality/Section 3: FPP 3.1. Manufacturing and marketing authorization 3.2. Pharmaceutical development 3.3. Formulation 3.4. Sites of manufacture 3.5. Manufacturing process Manufacturing process controls of Critical steps and intermediates 3.7. Process validation and Evaluation 3.8. Specifications for excipients Control of the FPP 3.10. Container/closure system (s) and other packaging 3.11. Stability testing
38 Generic Guide: Quality/Section 3: FPP
World Health Organization 20 April, 2017 Generic Guide: Quality/Section 3: FPP 3.12. Container labelling 3.13. Product information for health professionals 3.14. Patient information and package leaflet 3.15. Justification for any differences to the product in the country or countries issuing the submitted WHO-type certificate(s)
39 World Health Organization
20 April, 2017 Bioequivalence dossier (BE) requirements for the prequalification project
40 Generic Guide: BE Dossier requirements: general
5. Interchangeability 5.1 Bioequivalence study 5.2 Summary of pharmacology, toxicology* and efficacy of the product (expert reports) * not required anymore for artemisinines but for combinations
41 Generic Guide: BE Basic guidelines
World Health Organization 20 April, 2017 Generic Guide: BE Basic guidelines In vivo Bioequivalence studies are clinical trials: in accordance with the guidelines on Good Clinical Practice Good Manufacturing Practice Good Laboratory Practice
42 Generic Guide: BE Basic guidelines
Additional guidance WHO TRS No. 937, 2006, Annex 9 Guidelines for organizations performing in vivo bioequivalence studies. In: WHO Expert Committee on Specifications for Pharmaceutical Preparations. Fortieth report. Geneva, World Health Organization
43 Generic Guide: BE study report
The Bioequivalence study report should include information on: Ethics, Investigators and administrative structure Clinical phase of a study Bioanalytical method of study Pharmacokinetic and statistical analysis Study protocol
44 Generic Guide: BE study report
Complete structure is presented: Table of Contents Bioequivalence Trial Information Form (BTIF)
45 Generic Guide: BE dossier requirements SPC and PIL
The “generic” SPC + PIL = innovator SPC + PIL
46 Overview of Dossier Requirements and Guidelines (3)
Main guideline: Supplements Supplement 1 Guideline on Submission of Documentation for Prequalification of Multi-source (Generic) Finished Pharmaceutical Products (FPPs) Used in the Treatment of HIV/AIDS, Malaria and Tuberculosis Dissolution Testing for use from July 2005 (CPH25)
47 Supplement 1: Dissolution Testing Definitions
Immediate release: means that 75% of the API is dissolved within 45 minutes. Rapidly dissolving: means that 85% of the API is dissolved within 30 minutes. Very rapidly dissolving: means that 85% of the API is dissolved within 15 minutes
48 Supplement 1: Dissolution Testing Choice of Dissolution Media
Prescribes media to be considered for immediate release products during development studies: pH 6.8 buffer (or simulated intestinal fluid without enzymes) pH 4.5 buffer pH 1.2 buffer (or simulated gastric fluid without enzymes) or 0.1 M hydrochloric acid. Water may be considered as an additional medium Recommends testing intervals in the above media for purposes of generation of dissolution profiles: 10, 15, 20, 30 and 45 minutes.
49 Supplement 1: Dissolution Testing Role in Pharmaceutical Development
Enables: Selection of the formulation, by comparison of the dissolution profiles with that of the innovator product. This is to maximize the chances of bioequivalence. Comparison of the release properties of the pivotal batches to demonstrate in vitro similarity, which is considered essential for retention of efficacy and safety. Note that bioequivalence studies are done normally only once on a pivotal batch during development – it must therefore be demonstrated that the product retains the release characteristics up to and during commercial production. The selection of the dissolution specifications (conditions and acceptance criteria) for product release and stability study purposes. A dissolution specification should be discriminating, implying that it should be able to detect inadequate release properties of the commercial batches. Post-approval amendment application. If the amendment is of a major nature and requires bioequivalence studies, in vitro data may be acceptable, provided that (1) the profiles of the amendment batch and the current batch are similar and (2) that the dissolution study design is acceptable (preferably the three media and short interval multipoint).
50 Supplement 1: Dissolution Testing Scenarios
If both the test and reference product show more than 85% dissolution within 15 minutes, the profiles are considered similar (no calculations required). Calculate the f2 value. If f2 ≥ 50, the profiles are normally regarded similar. Note that only one measurement should be considered after 85% dissolution of both products has occurred and excluding point zero. The evaluation of similarity is based on the conditions of a minimum of three time points (zero excluded) 12 individual values for every time point for each formulation not more than one mean value of > 85% dissolved for each formulation that the standard deviation of the mean of any product should be less than 10% from second to last time point.
51 Supplement 1: Dissolution Testing Setting specifications
The specifications for the in vitro dissolution of the product should be derived from the dissolution profile of the batch that was found to be bioequivalent to the reference product and would be expected to be similar to those of the reference product. For immediate release products, if the dissolution profile of the test product is dissimilar compared to that of the reference product and the in vivo data remains acceptable, the dissolution test method should be re-evaluated and optimised. In case that no discriminatory test method can be developed which reflects in vivo bioequivalence, a different dissolution specification for the test product could be set.
52 Overview of Dossier Requirements and Guidelines (4)
Main guideline: Supplements Supplement 2 Guideline on Submission of Documentation for Prequalification of Multi-source (Generic) Finished Pharmaceutical Products (FPPs) Used in the Treatment of HIV/AIDS, Malaria and Tuberculosis Extension of the WHO List of Stable (not easily degradable ARV) APIs (for stability testing) for use from July 2005 (CPH25)
53 Supplement 2: Stability Data for stable APIs List of stable APIs
Abacavir Amodiaquine Didanosine Efavirenz Ethambutol 2HCl Ethionamide Isoniazid Lamivudine Lumefantrine Mefloquine Nevirapine Prothionamide Pyrazinamide Pyrimethamine Stavudine Sulfadoxine Zidovudine
54 Supplement 2: Stability Data for stable APIs Evaluation Criteria for APIs
A two (2) years’ re-test period may be accepted on the basis of six (6) months’ accelerated and six (6) months’ long-term stability studies, if: Both the accelerated and the long-term stability data show so little degradation and so little variability. The Applicant undertakes in writing to continue long-term testing of the API under evaluation for a period of time sufficient to cover the whole proposed retest date (NLT 24 months) and to report any out-of-specification results immediately to WHO.
55 Supplement 2: Stability Data for stable APIs Singe API-FPPs
A two (2) years’ shelf life may be accepted for the single-API FPPs on the basis of six (6) months’ accelerated and six (6) months’ long-term stability studies, if: The API is known to be stable (not easily degradable) Supporting data indicates that similar formulations have been assigned a shelf-life of 24 months or more; The manufacturer will continue to conduct real-time studies until the proposed shelf-life has been covered, and the results obtained will be submitted to the registration authority. Additional requirements: The tentative shelf life applies only to hard capsules and tablets containing only one of the above-listed APIs. The applicant should provide evidence that the primary packing material protects the FPP against humidity and light, when applicable. Both the accelerated and the long-term stability data should show so little degradation and so little variability The Applicant undertakes in writing to continue long-term testing of the FPP under evaluation for a period of time sufficient to cover the whole proposed retest date (NLT 24 months) and to report any out-of-specification results immediately to WHO.
56 Supplement 2: Stability Data for stable APIs FDC Capsules & Tablets
A two (2) years’ shelf life for FDC FPPs may be accepted on the basis of six (6) months’ accelerated and six (6) months’ long-term stability studies, if: All conditions for Single API-FPPs are met the compatibility of the APIs with each other is demonstrated by stress testing. Any evaluation should take into account the assay and the degradation or reaction products. Supporting HPLC chromatograms should be submitted.
57 Overview of Dossier Requirements and Guidelines (5)
Guideline FPPs approved by ICH & associated DRAs Guide on Submission of Documentation for Prequalification of Finished Pharmaceutical Products (FPPs) used in the treatment of HIV/AIDS, malaria and tuberculosis and approved by Drug Regulatory Authorities (DRAs) in the International Conference on Harmonization (ICH) region and associated countries, including inter alia the EU, Japan and USA
58 FPPs approved by ICH & associated DRAs
A covering letter with: An original or certified copy of WHO-type Certificate of a Pharmaceutical Product issued by one of the regulatory authorities in the ICH region and associated countries, together with the approved summary of product characteristics (SmPC), or an equivalent thereof. Assessment report(s) issued by a DRA in the ICH region and associated countries. European Public Assessment Report (EPAR) is also acceptable. If the composition/formulation, strength, specifications, etc. are different from the product for which the WHO-type Product Certificate(s) was issued, then arguments and/or data to support the applicability of the certificate(s) — demonstration of pharmaceutical equivalence and bioequivalence should be submitted. If the primary packaging material of the product is different from the one approved by the drug regulatory authorities of the ICH regions and associated countries, then stability testing data should be submitted. Provide a sample of the FPP(s) to enable visual inspection of the FPP(s). Attach certificate of analysis. Variations to the terms of prequalification of a FPP should be implemented by the applicant only after the proposed changes have been evaluated and approved by WHO.
59 Overview of Dossier Requirements and Guidelines (6)
Guidelines: Fixed-Dose Combinations (FDCs) WHO Expert Committee on Specifications for Pharmaceutical Preparations. 2005 Thirty-ninth report (WHO Technical Report Series, No. 929), Annex 5: Guidelines for registration of fixed-dose combination medicinal products
60 Guidelines: FDCs Definitions
Fixed-dose combination (FDC) A combination of two or more actives in a fixed ratio of doses. This term is used generically to mean a particular combination of actives irrespective of the formulation or brand. It may be administered as single entity products given concurrently or as a finished pharmaceutical product. Fixed-dose combination finished pharmaceutical product (FDC-FPP) A finished pharmaceutical product that contains two or more actives.
61 Guidelines: FDCs The Four Scenarios
Scenario 1. The new FDC-FPP contains the same actives in the same doses as an existing FDC-FPP; that is it is a “generic” of the existing FDC-FPP; they are “multisource” products. The quality, safety and efficacy of the existing product have been established. Scenario 2. The new FDC-FPP contains the same actives in the same doses as an established regime of single entity products, and the dosage regimen is the same. Alternatively the established regime may involve combinations of single entities and FDCs, for example, a single entity FPP combined with an FDC-FPP that contains two actives. In all cases, the established regime has a well-characterized safety and efficacy profile, and all of the FPPs used in obtaining clinical evidence have been shown to be of good quality. Scenario 3 The new FDC-FPP combines actives that are of established safety and efficacy but have not previously been used in combination for this indication. The new FDC-FPP comprises a combination for which safety and efficacy have been established, but that will be used in a different dosage regimen. Scenario 4. The new FDC-FPP contains one or more new chemical entities.
62 Guidelines: FDCs Registration Requirements (1)
Data requirements for marketing authorization of FDC-FPPs depend broadly on the scenario into which the application falls. Issues that are specific to the development of FDC-FPPs include: Chemical and physicochemical compatibility of the APIs in an FDC with one another as well as with possible excipients. The degradability of each API under stress conditions in the presence of the others. Uniformity of content of each active prior to compression (tablets) or filling (for instance capsules, sachets and suspension dosage forms). This study determines whether mixing during manufacture is adequate.
63 Guidelines: FDCs Registration Requirements (2)
Analytical procedures. Validation should be conducted for each active in the presence of the others and in the presence of related synthesis (process) impurities and potential degradation products. In the case of high-performance liquid chromatography (HPLC), possible interference by degradation products in the assay of the active can usually be controlled by peak purity testing. The dissolution rate of each active in pilot formulations. Multipoint limits should normally be established for routine quality control of each active. For some FDC-FPPs, different dissolution media may be acceptable for the different actives. Different assay procedures may be necessary for the different actives in the finished product, and for different purposes (e.g. dissolution testing may be needed rather than stability testing).
64 Guidelines: FDCs Registration Requirements (3)
6.3.3 For solid dosage forms: a test and limit for content uniformity should be applied to any active that is present at a weight of ≤25mg or when the API comprises 25% or less of a dosage unit. when any one API is present at less than 25 mg or less than 25% of the weight of a dosage unit, all of the actives are subjected to content uniformity testing. a test and limit for mass variation should be applied if all of the actives are present in a solid dosage form at a weight of greater than 25mg and greater than 25% of the weight of a dosage unit.
65 Guidelines: FDCs Registration Requirements (4)
6.3.4 Acceptance criteria for impurities in FDC-FPPs should be expressed with reference to the parent API (and not with reference to the total content of APIs). If an impurity results from reaction between two APIs, its acceptance limits should be expressed in terms of the API that represents the worst case. If available, a reference standard should be used to quantify the degradation product in percentage mass/mass with respect to the parent API. Note: there should be an approximate mass balance. Together with the remaining active, degradants expressed with reference to the parent compound should sum to approximately 100% of initial strength. 6.3.5 The specifications and defining characteristics of the product should be based on the most vulnerable active. For example expiry dates should be based on the stability of the least stable active.
66 Guidelines: FDCs Registration Requirements (5)
Bioequivalence for FDC-FPPs: Comparison with an existing combination separate active APIs (drug)
67 Overview of Dossier Requirements and Guidelines (7)
ICH guidelines ICH guidelines are used when a quality aspect cannot be (fully) assessed by the WHO guidelines, for instance: Q3A(R). Impurities in new drug substances Q3B(R). Impurities in new drug products Q3C. Impurities: Guideline for residual solvents Q6A. Specifications: Test procedures and acceptance criteria for new drug substances and new drug products: chemical substances (with decision trees)
68 Overview of Dossier Requirements and Guidelines (8)
GUIDANCE ON VARIATIONS TO A PREQUALIFIED PRODUCT DOSSIER The prequalification process is dynamic, taking into account that changes to the original dossier may become necessary during the lifetime of the product Any changes or variations may involve administrative and/or more substantial changes and are subject to approval within the prequalification program Where a variation requires consequential revision of the Summary of Product Characteristics (SmPC), labelling and package leaflet/insert, this is considered as part of the variation. Whenever FPPs have been prequalified on the basis of approval by a drug regulatory authority of the ICH region and associated countries (Innovator Products or Generic Products) subsequent variation applications are also to be approved by these drug regulatory authorities and WHO should be notified about the approval of the changes.
69 Variation Guideline (2)
ANNEX I List of minor changes The conditions which must apply are stipulated The relevant part of the dossier to be resubmitted or updated, with the documentation required, is listed ANNEX II Lists major changes in general: Major changes exceed the scope of minor changes as listed in Annex I, e.g. they exceed/do not comply with the conditions to be fulfilled along with the change: Change in the manufacturing process of the API Change in the composition of the finished product Change of immediate packaging of the product ANNEX III Lists types of changes which may require a new application: Changes to the API (±type, quantity) Changes to the pharmaceutical form/dosage form Changes in the route of administration
70 Variation Guideline (3)
Approval of changes Applications for minor changes that are classified notifications (N) must provide evidence to fulfil the conditions and documentation requirements as listed. Within a period of three months these notifications will be evaluated by WHO and can be considered approved if no correspondence by WHO with the applicant has been initiated within that time. For all other change applications that are not considered as notifications, prior approval by WHO is always necessary before the changes can be implemented. Certain changes are so fundamental that they alter the terms of the prequalified dossier and consequently cannot be considered as a change. For these cases a new dossier must be submitted (Annex III). All parts of the dossier that are affected by a variation are to be resubmitted according to the structure of the Pharmaceutical Quality Information Form (PQIF)1
71 Closing remarks The dossier submitted must conform to the requirements set out in the current WHO guidelines, as posted on web The assessment of quality and safety/efficacy data presented is based on the current WHO guidelines ICH guidelines are used when a quality aspect cannot be assessed by the WHO guidelines The quality assessment includes variations or changes to already prequalified products
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION. WHO FUNCTION.
WORLD HEALTH ORGANIZATION
Presentation Transcript
WHO FUNCTION • WHO is the directing and coordinating authority for health within the United Nations system. It is responsible for providing leadership on global health matters, shaping the health research agenda, setting norms and standards, articulating evidence-based policy options, providing technical support to countries and monitoring and assessing health trends. • In the 21st century, health is a shared responsibility, involving equitable access to essential care and collective defence against transnational threats.
GOVERNANCE/WORLD HEALTH ASSEMBLY • The World Health Assembly is the supreme decision-making body for WHO. It generally meets in Geneva in May each year, and is attended by delegations from all 193 Member States. Its main function is to determine the policies of the Organization. The Health Assembly appoints the Director-General, supervises the financial policies of the Organization, and reviews and approves the Proposed programme budget. It similarly considers reports of the Executive Board, which it instructs in regard to matters upon which further action, study, investigation or report may be required.
THE EXECUTIVE BOARD • The Executive Board is composed of 34 members technically qualified in the field of health. Members are elected for three-year terms. The main functions of the Board are to give effect to the decisions and policies of the Health Assembly, to advise it and generally to facilitate its work. • The Secretariat of WHO is staffed by some 8000 health and other experts and support staff on fixed-term appointments, working at headquarters, in the six regional offices, and in countries. • The Organization is headed by the Director-General, who is appointed by the Health Assembly on the nomination of the Executive Board.
WHO AGENDA • “I WANT MY LEADERSHIP TO BE JUDGED BY THE IMPACT OF OUR WORK ON TWO POPULATIONS : WOMEN AND THE PEOPLE OF AFRICA” …DR MARGARET CHAN, DIRECTOR GENERAL.
WHO: PROMOTING DEVELOPMENT • During the past decade, health has achieved unprecedented prominence as a key driver of socioeconomic progress, and more resources than ever are being invested in health. Yet poverty continues to contribute to poor health, and poor health anchors large populations in poverty. Health development is directed by the ethical principle of equity: Access to life-saving or health-promoting interventions should not be denied for unfair reasons, including those with economic or social root. • WHO activities aimed at health development give priority to health outcomes in poor, disadvantaged or vulnerable groups. • CORNERSTONES OF THE HEALTH AND DEVELOPMENT AGENDA • Attainment of the health-related Millennium Development Goals, • Preventing and treating chronic diseases and • Addressing the neglected tropical diseases.
WHO: FOSTERING HEALTH SECURITY • 2. Fostering health security • Shared vulnerability to health security threats demands collective action. One of the greatest threats to international health security arises from outbreaks of emerging and epidemic-prone diseases. Such outbreaks are occurring in increasing numbers, fuelled by such factors as rapid urbanization, environmental mismanagement, the way food is produced and traded, and the way antibiotics are used and misused. The world’s ability to defend itself collectively against outbreaks has been strengthened since June 2007, when the revised International Health Regulations came into force.
WHO: STRENGTHENING HEALTH SYSTEMS • 3. Strengthening health systems • For health improvement to operate as a poverty-reduction strategy, health services must reach poor and underserved populations. Health systems in many parts of the world are unable to do so, making the strengthening of health systems a high priority for WHO. Areas being addressed include the provision of adequate numbers of appropriately trained staff, sufficient financing, suitable systems for collecting vital statistics, and access to appropriate technology including essential drugs.
WHO: HARNESSING RESEARCH… • 4. Harnessing research, information and evidence • Evidence provides the foundation for setting priorities, defining strategies, and measuring results. WHO generates authoritative health information, in consultation with leading experts, to set norms and standards, articulate evidence-based policy options and monitor the evolving global heath situation.
WHO: ENHANCING PARTNERSHIP • 5. Enhancing partnerships • WHO carries out its work with the support and collaboration of many partners, including UN agencies and other international organizations, donors, civil society and the private sector. WHO uses the strategic power of evidence to encourage partners implementing programmes within countries to align their activities with best technical guidelines and practices, as well as with the priorities established by countries.
WHO: GLOBAL BURDEN OF DISEASE Global Burden of Disease (GBD) 2010 study WHO is participating in a multi-center collaboration to revise and update global estimates of burden of disease, injury and risk factors for the years 1990 and 2005. Many WHO staff members are involved this project: participating in core planning of the project; providing access to WHO databases and providing expertise on specific diseases, injuries, and risk factors. • The Bill & Melinda Gates Foundation has provided funding for a new round of the Global Burden of Disease study, the GBD 2010 study, to be published in 2011. The study is led by the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, with key collaborating institutions including WHO, Harvard University, Johns Hopkins University and the University of Queensland. The GBD 2010 study will develop improved methods to make full use of the increasing amount of health data, particularly from developing countries, and will include a comprehensive and consistent revision of disability weights. The study will also assess trends in the Global Burden of Disease from 1990 to 2005.
WHO ON ALCOHOL • Although alcohol consumption has occurred for thousands of years, many of the varied health effects have been discovered fairly recently. Alcohol consumption has health and social consequences via intoxication (drunkenness), dependence (habitual, compulsive and long-term drinking), and other biochemical effects. In addition to chronic diseases that may affect drinkers after many years of heavy use, alcohol contributes to traumatic outcomes that kill or disable at a relatively young age, resulting in the loss of many years of life to death or disability. There is increasing evidence that besides volume of alcohol, the pattern of the drinking is relevant for the health outcomes. Overall there is a causal relationship between alcohol consumption and more than 60 types of disease and injury. Alcohol is estimated to cause about 20-30% worldwide of oesophageal cancer, liver cancer, cirrhosis of the liver, homicide, epilepsy, and motor vehicle accidents.
WHO ON ALCOHOL • Globally alcohol consumption has increased in recent decades, with all or most of that increase in developing countries. This increase is often occurring in countries with little tradition of alcohol use on population level and few methods of prevention, control or treatment. The rise in alcohol consumption in developing countries provides ample cause for concern over the possible advent of a matching rise in alcohol-related problems in those regions of the world most at risk. • Worldwide alcohol causes 2.5 million deaths (3.8 % of total) and 69.4 million (4.5 % of total) of Disability-Adjusted Life Years (DALYs). Unintentional injuries alone account for about one third of the 2.5 million deaths, while neuro-psychiatric conditions account for close to 40% of the 69.4 million DALYs. The burden is not equally distributed among the countries.
WHO:HEALTH RISK OF MOBILE PHONES Given the immense number of people who use mobile phones, even a small increase in the incidence of adverse effects on health could have major public health implications. • Because exposure to the radiofrequency (RF) fields emitted by mobile phones is generally more than a 1000 times higher than from base stations, and the greater likelihood of any adverse effect being due to handsets, research has almost exclusively been conducted on possible effects of mobile phone exposure. • Research has concentrated on the following areas: • cancer • traffic accidents • electromagnetic interference • other health effects. • Conclusions: No recent national or international reviews have concluded that exposure to the RF fields from mobile phones or their base stations causes any adverse health consequence. However, areas have been identified by the WHO’s EMF Project for further research to better assess health risks and have led to over US$ 250 million in research worldwide to study RF effects on health. It will take about 2-3 years for the required RF research to be completed, evaluated and to publish an updated WHO health risk assessment.
WHO ON CLONING • 1. What is a clone? • The term clone, from the Greek for “twig,” denotes a group of identical entities; in recent years, “clone” has come to mean a member of such a group and, in particular, an organism that is a genetic copy of an existing organism. The term is applied by scientists not only to entire organisms but to molecules (such as DNA) and cells.
WHO ON CLONING • Why did scientists develop cloning techniques? • Scientists were initially interested in SCNT as a means of determining whether all the genes in an organism’s genome remain functional even after most of them have been switched off as a developing organism’s cells assume their specialized functions as blood, bone, muscle, and so forth. The ability of scientists to stimulate the DNA in a nucleus from a fully differentiated cell to revert to a condition comparable to the DNA in a newly fertilized egg and to begin the process of embryonic development demonstrated that all the genes remain viable in differentiated cells even though only a few genes are actually expressed in each cell. Commercial interest in animal cloning centres on replicating large numbers of genetically identical animals, especially those derived from a progenitor which has been modified genetically. In this fashion, mice or other laboratory animals that exhibit particular conditions can be created for specialized studies, or herds of farm animals (such as goats, sheep, or cows) can be created all of whom produce pharmaceutically useful proteins in their milk.
WHO ON CLONING • How does “Dolly type” cloning occur? • To produce Dolly, the researchers used an improved version of the technique of somatic-cell nuclear transfer (SCNT) first used 40 years ago in research with tadpoles and frogs. SCNT begins with an adult somatic cell, for example, a skin cell. “Adult” means a fully differentiated cell from an organism that had passed the embryonic stage of development, and “somatic” denotes a body cell (rather than an egg or sperm cell), which possesses the full complement of chromosomes, rather than the half contained in gametes. The nucleus from the somatic cell is transferred to an enucleated egg (that is, one from which the nucleus has been removed). The egg is then activated with electric current or chemicals in order to stimulate it to divide. When the blastocyst stage has been reached, the embryo is transferred into the uterus of a female host, where – if implantation occurs – it can lead to a pregnancy and eventually to the birth of an individual that carries the same nuclear genetic material as the donor of the adult somatic cell. Animals created through SCNT are not precise genetic copies of the donors of their nuclear DNA, however, since a small amount of DNA resides in the mitochondria outside the egg’s nucleus; mitochondrial DNA is normally passed on to children only from their mothers. Since a clone would derive its mitochondrial DNA from the egg, not from the donor of the nucleus, the clone and its progenitor would be genetically identical only if the egg came from the progenitor or from the same maternal line.
WHO ON CLONING What is WHO’s position on cloning to replicate a human being (“human reproductive cloning”)? • The Member States of the World Health Organization (WHO) consider that developments in human reproductive cloning have unprecedented ethical implications and raise serious concerns for the safety of individuals and subsequent generations of human beings. The World Health Assembly has therefore resolved that the use of cloning «for the replication of human individuals is ethically unacceptable and contrary to human dignity and integrity.»
WHO ON CLONING What justifications are offered for non-reproductive human cloning? • Scientists engaged in cloning for research argue that it presents a unique method for studying genetic changes in cells derived from patients suffering from such diseases as Parkinson’s disease, Alzheimer’s disease, and diabetes. Scientists who are interested in such research look ahead to the day when they believe that embryonic stem cells will be used to assist drug development and evaluation, for diagnostic purposes, and to create cells and tissues for transplantation. For the latter, if the stem cells used in transplantation were derived from embryos cloned from the patient needing the transplant, they might be less subject to rejection than cells, tissues or organs from another person, since the DNA in the cloned cells would be nearly identical to the patient’s own.
Презентация была опубликована 6 лет назад пользователемАнтон Пономарев
Похожие презентации
1 WORLD TRADITIONAL MEDICAL SYSTEM IN RUSSIA
3 SCIENTIFIC MEDICINE Includes a system of medical knowledge, based on the data of scientific and technological progress associated with the experiments, which resulted in verified empirical knowledge and philosophical ideas are scientifically sound concepts, hypotheses, theories. TRADITIONAL MEDICINE is a therapeutic direction, using methods that came to us from antiquity, who had not lost its significance today. Traditional medicine has a deep history and is based on a large centuries-old experience of the people.
4 In our country (Russia) Traditional medicine Ia a set of knowledge, skills, abilities, including those based on practice and observation handed down from generation to generation orally or in writing, and do not always have a scientific rationale and logical explanation used in the processes of prevention, diagnosis and treatment of diseases and medical rehabilitation.
5 The types of traditional medicine include: Reflexology Manual therapy Phytotherapy (Herbal medicine) Naturopathy Homeopathy Medical massage Traditional diagnosis Traditional healing system Folk medicine (syn. healing)
7 Reflexology (reflexotherapy, acupuncture) system of diagnostic and therapeutic methods based on the estimation of parameters of peripheral reflex zones and impacts on them with a view to the regulation of functional systems
8 Acupuncture methods Depending on the impact tool and method acupuncture (needling) thermopuncture electropuncture electroacupuncture laserpuncture elektromagnitopuncture lightpuncture phonopuncture farmakopuncture acupressure apirefleksoterapiya (Bee treatment) Hirudorefleksoterapiya (Leech treatment) Vakuumrefleksoterapy (Cap treatment) energy-RT (multiresonance and bioresonance) combined methods of acupuncture
9 By localisation Corporal Acupuncture Corporal Acupuncture Reflexology on mikroacupuncture systems Reflexology on mikroacupuncture systems Auricular Acupuncture Auricular Acupuncture Cranial (of scalp) Cranial (of scalp) Micro-acupuncture system of hand and foot (Su- Jok RT, mano-, pedo-RT) Micro-acupuncture system of hand and foot (Su- Jok RT, mano-, pedo-RT) Nasal reflexology (exo and endonasal) Nasal reflexology (exo and endonasal) Oral reflexology Oral reflexology Mikroacupuncture using other systems. Mikroacupuncture using other systems. Now we can discuss AURICULAR ACUPUNCTURE & DIAGNOSE METHOD. Now we can discuss AURICULAR ACUPUNCTURE & DIAGNOSE METHOD.
15 Traditional diagnosis set of diagnostic methods permitted for medical use in the Russian Federation in accordance with established procedure, which are based on laws, principles and presentation of various traditional medical systems. Traditional diagnosis is based on getting to the surface of the body of information about the state of his internal organs and systems. set of diagnostic methods permitted for medical use in the Russian Federation in accordance with established procedure, which are based on laws, principles and presentation of various traditional medical systems. Traditional diagnosis is based on getting to the surface of the body of information about the state of his internal organs and systems.
18 Traditional healing system group of species of traditional medical activities based on domestic and foreign traditional medical systems, permitted for medical use (registered) Ministry of Health in the prescribed manner and subject to licensing in the Russian Federation. group of species of traditional medical activities based on domestic and foreign traditional medical systems, permitted for medical use (registered) Ministry of Health in the prescribed manner and subject to licensing in the Russian Federation.
19 Energoinformacy combination of methods, which are based on the impact of the human biofield. combination of methods, which are based on the impact of the human biofield. Impact on the human body for the purpose of diagnosis and treatment of diseases. Impact on the human body for the purpose of diagnosis and treatment of diseases.
World Health Organization
World Health Organization. Collaborating Center in Reproductive Health. Emory University Woodruff Health Sciences Center The National Centers for Disease Control and Prevention (CDC) Georgia Department of Human Resources Division of Public Health. Alfred W. Brann, Jr., MD, Director
World Health Organization
Presentation Transcript
World Health Organization Collaborating Center in Reproductive Health Emory University Woodruff Health Sciences Center The National Centers for Disease Control and Prevention (CDC) Georgia Department of Human Resources Division of Public Health Alfred W. Brann, Jr., MD, Director Woodruff Health Sciences Center Emory University Brian McCarthy, MD, Principal Investigator The National Centers for Disease Control and Prevention
Collaborating Center in Reproductive Health Vision Every woman should have the opportunity, if she desires, to experience a wanted pregnancy that results in a full-term normal baby, and following childbirth that she is alive and healthy and her infant is healthy and safe in the context of family and community.
Women’s Health • Education • Gender Issues • Religion • Culture/Society/Family
Women’s Health • Political Stability • Economy/Financial Security • Health Care System • Government Structure
NEED • More than 50% of the infant deaths annually occur during the first week afterbirth. • This is largely a consequence of a poorly functioning health care delivery system that results in the poor health of women, and poorly managed pregnancies and births.
NEED • Knowledge and skills exist today that can reduce by some 50% the excessive rates of both maternal and infant mortality. • This is possible if there are appropriate attitudes and if there is a functioning modern maternal and perinatal health care delivery system.
Collaborating Center in Reproductive Health Mission …to serve the people who serve the people. by enabling health professionals in ministries of health, departments of health, or universities to build a foundation of sustainable knowledge and skills in health services research through education using a state or country-specific health project at the level of the Health District.
The Balashikha Project MISSION • To dramatically improve U.S.-Russia relations through a public/private initiative addressing “the most acute problem facing Russia – “its declining population” (V. Putin, 2001).
The Balashikha Project WHY • Russia’s new births are not at replacement levels. Outmigration, decreasing adult lifespan (less than 59 years) and high infant mortality all contribute to its declining population.
The Balashikha Project WHAT • Create a sustainable reduction in population decline by decreasing infant mortality by 50% in a significant sized health region in Russia.
The Balashikha Project WHERE • City of Balashikha and the Moscow Oblast, Russia
The Balashikha Project HOW • Establish a maternal and perinatal health care delivery system (using an internationally integrated set of health strategies) in the Moscow Oblast to significantly reduce infant mortality, and serve as a model for the Russian Federation.
Levels of the Health Care Delivery System (HCDS) Informal • Skill • Knowledge • Attitude • Resources • Management • Manpower • Materials • Money Formal Inter- sectoral
The Balashikha Project • Components of a Maternal and Perinatal Health Care Delivery System • The Moscow Region Perinatal Center • Evidence-based Perinatal Guidelines • Modern Perinatal Surveillance System
The Balashikha Project • Components of a Maternal and Perinatal Health Care Delivery System • Continuous Quality Improvement Program • International Public Health Practice • Postgraduate Education-Distance Learning • Public Policy and Legal Assistance
The Balashikha Project Microsoft Health Care Application SoftwareVertical Health Initiatives such as Word, Excel, etc. such as HIV/AIDS, Polio, etc. Operating SystemsHealth Care DeliverySystem on which all Application on which all Vertical Health Software is placedInitiatives are placed.
The Balashikha Project WHO • The following partners have brought about the Balashikha Project.
The Balashikha Project WHY SHOULD AMERICANS HELP RUSSIA? • Good health is a bedrock issue for building a democratic society and is essential for economic development. • Russia, with an educated and aspiring domestic workforce, can become one of our most important trading partners. • Russia is a strong ally of the U.S. in the war on terrorism. • Our support creates another level of mutual trust and respect.
The Balashikha Project “I thinknothing our generation could do is more important than getting Russia into the fold of ‘successful’ countries, and nothing worse could happen than to lose it.” Dr. Howard Mette, Rotarian and CCI/PEP Volunteer, Youngstown, Ohio, 2000
NEED The status of the health of the infant is dependent on the status of health of the woman and the pregnant woman.
NEED • The vast majority of men and women wish to have children. • All societies depend on their next generation for their survival, well being, success and social security.
NEED • Pregnancy and childbirth are the only normal physiologic processes associated with a risk of death. • Women (unlike men) are exposed to this risk of death during pregnancy and childbirth.
NEED • Finally, the health care system supporting pregnancy and childbirth is the one through which we must all pass, whether it is a good or bad system.
NEED • Problems in reproductive health affect many lives and families. • The morbidity and disability in both mother and child who receive suboptimal health care have devastating and permanent consequences. • These individual consequences further adversely impact society and its ability to not just survive but thrive.
Health Services Research- The systematic study of whether current medical and other relevant knowledge is effectively used to improve the health of a community under an existing set of conditions.
DATA…. INFORMATION…. INTERVENTION
Interventions for Reducing Mortality • Women’s and Maternal Health • Maternal and Fetal Care • Neonatal Care • Infant Care
The “Opportunity Gap”- The potential for reduction in excessive mortality based on a comparison between rates already achieved by one sub-population in a defined geographical area with those experienced by the remaining population.
NEED • The problems leading to high rates of mortality and morbidity are not randomly distributed – women who are economically and intellectually disadvantaged have, along with their infants, the highest mortality rates, regardless of whether they live in a third-world country or in third-world conditions in a developed country.
NEED • Any further significant reduction in infant mortality will need to focus on improving the health of the woman. • This will involve (1) changing the frequency of premature infant births, and (2) decreasing neonatal deaths, with a particular focus on the early neonatal period (1st week).
Georgia’s Six Perinatal Regions Hospital Perinatal Center
Management Process Performance Assessment Problem Definition Monitoring & Evaluation Intervention Are we doing the right thing? Are we doing things right?
The Management Cycle in Maternal and Child Health Care PROBLEM DEFINITION A Standard • High Risk vs. Lower Risk • Problem Description • Identification of Risk Factors • Identification of High Risk Groups
The Management Cycle in Maternal and Child Health Care Performance Evaluation Guidelines • Four Essential Questions to Be Asked: • What is supposed to happen? • What do people think is happening? • What do people say is happening? • What is really happening?
The Management Cycle in Maternal and Child Health Care INTERVENTION • What are they doing? vs What should they do? • Coverage • Referral Pattern • Matching Skill • Altering Risk Factors • Training/Education • Planning/Policy • The five R’s • The right person • At the right place • At the right time • Doing the right thing • Doing it right
The Management Cycle in Maternal and Child Health Care MONITORING & EVALUATION A Standard • Evaluation = Measurement & Comparison • Outcome • Process • Community Education • Training • Use of Appropriate Technology
Surveillance- a dynamic process which collects, analyzes, and responds to data on the occurrence and distribution of events in a defined population within a geographical area.
You can not manage. What you do not measure.
We are data rich… But information poor.
DATA…. INFORMATION…. INTERVENTION
Total Cohort Accountability • “Every pregnancy counts… • so account for every pregnancy.” • “Every newborn has weight… • so weigh every newborn.”
Conceptual Framework for Action Trigger Symbols • Model for the HCDS • Perinatal Surveillance Principles • Management Process
NEED • The World Bank (in a 1993 publication ―Investing in Health) described GOOD HEALTH as essential for economic development throughout the world and made two major recommendations.
Презентация была опубликована 8 лет назад пользователемРодион Осташков
Похожие презентации
Презентация на тему: » 7 апреля – Всемирный день здоровья. Всемирный день здоровья (World Health Day) отмечается ежегодно 7 апреля в день создания в 1948 году Всемирной организации.» — Транскрипт:
1 7 апреля – Всемирный день здоровья
2 Всемирный день здоровья (World Health Day) отмечается ежегодно 7 апреля в день создания в 1948 году Всемирной организации здравоохранения (World Health Organization, WHO). За время, прошедшее с того исторического момента, членами Всемирной организации здравоохранения (ВОЗ) стали 194 государства мира.
3 Ежегодное проведение Дня здоровья вошло в традицию с 1950 года. Мероприятия Дня проводятся для того, чтобы люди могли понять, как много значит здоровье в их жизни. А здравоохранительные организации призваны решить вопрос, что им нужно сделать, чтобы здоровье людей во всем мире стало лучше. Каждый год Всемирный день здоровья посвящается глобальным проблемам, стоящим перед здравоохранением планеты и проходит под разными девизами.
4 В 2009 году главной темой Всемирного дня здоровья стала безопасность медицинских учреждений и готовность работников здравоохранения оказывать помощь людям, пострадавшим в чрезвычайных ситуациях. Во всем мире прошли мероприятия, посвященные пропаганде безопасных конструкций медицинских учреждений и улучшению готовности к чрезвычайным ситуациям.
5 В 2010 году девизом были выбраны слова «1000 городов – 1000 жизней», потому что в фокусе Дня находились урбанизация и здоровье. Во всем мире проводились мероприятия, направленные на то, чтобы в городах были созданы условия для проведения оздоровительных мероприятий на улицах. Проводились уличные соревнования, репортажи о которых публиковались с целью пропаганды здорового образа жизни в городах.
6 В 2011 году темой, обсуждаемой на мероприятиях Дня, стала устойчивость к антимикробным препаратам. Медицина обеспокоена тем, что резистентность (от англ. resistance, устойчивость) бактериальных агентов инфекционных заболеваний к антибиотикам становится одной из основных причин,ограничивающих эффективность антибактериальной терапии.Во Всемирный день здоровья 2011 г. Всемирная организация здравоохранения призывает ввести в действие комплекс из шести политических мер по борьбе с устойчивостью к противомикробным препаратам во всех странах. В 2011 году темой, обсуждаемой на мероприятиях Дня, стала устойчивость к антимикробным препаратам. Медицина обеспокоена тем, что резистентность (от англ. resistance, устойчивость) бактериальных агентов инфекционных заболеваний к антибиотикам становится одной из основных причин,ограничивающих эффективность антибактериальной терапии.Во Всемирный день здоровья 2011 г. Всемирная организация здравоохранения призывает ввести в действие комплекс из шести политических мер по борьбе с устойчивостью к противомикробным препаратам во всех странах.
7 В 2012 году темой Всемирного дня здоровья была – «Старение и здоровье», а лозунг этого дня – «Хорошее здоровье прибавляет жизни к годам». Внимание направлено на то, как хорошее здоровье на протяжении всей жизни может помочь людям пожилого возраста вести полноценную и продуктивную жизнь и оставаться полезными для своих близких и общества. Независимо от того, где мы живем, старение касается каждого из нас – молодых и пожилых, мужчин и женщин, богатых и бедных. В 2012 году темой Всемирного дня здоровья была – «Старение и здоровье», а лозунг этого дня – «Хорошее здоровье прибавляет жизни к годам». Внимание направлено на то, как хорошее здоровье на протяжении всей жизни может помочь людям пожилого возраста вести полноценную и продуктивную жизнь и оставаться полезными для своих близких и общества. Независимо от того, где мы живем, старение касается каждого из нас – молодых и пожилых, мужчин и женщин, богатых и бедных.
8 В 2013 году темой Всемирного дня здоровья была выбрана гипертония. Гипертония, или высокое кровяное давление, повышает риск развития инфаркта, инсульта и почечной недостаточности и может также приводить к слепоте, аритмии и сердечной недостаточности. У около 40% взрослых людей в мире развивается гипертония. Но гипертонию можно предотвращать и лечить.
9 Борьба с болезнями зависит не только от работы врачей. Ее успех зависит от понимания и желания людей создать для себя более здоровое будущее, предотвратить разрушение собственной среды обитания и изменить свое поведение.
10 Ведите здоровый образ жизни
12 1.Существуют тысячи болезней, но здоровье бывает только одно. Л. Берне 2. Единственная красота, которую я знаю, это здоровье. Г. Гейне 3.Если не бегаешь пока здоров, придется побегать, когда заболеешь. Гораций 4. Жизнерадостность это не только признак здоровья, но еще и самое действенное средство, избавляющее от болезней. С. Смайлс 5.Человек любит поговорить о своих болезнях, а между тем это самое неинтересное в его жизни. А. П. Чехов 6.Девять десятых нашего счастья зависит от здоровья А. Шопенгауэр 7. Здоровый человек самое драгоценное произведение природы. Т. Карлейль
13 Книжная выставка к Всемирному дню здоровья
World health organization
World health organization. “WHO ” by anna spencer erickson. WHO was Established on April 7, 1948; now known as World Health Day. http:// ecigarettereviewsite.net /world-health-organization-wants-electronic-cigarettes-banned-because-they-normalize-smoking/.
World health organization
Presentation Transcript
World health organization “WHO” by anna spencer erickson
WHO was Established on April 7, 1948; now known as World Health Day http://ecigarettereviewsite.net/world-health-organization-wants-electronic-cigarettes-banned-because-they-normalize-smoking/
WHO’s role is to Govern global health and disease and Establish and monitor global health standards http://newshopper.sulekha.com/margaret-chan_photo_1106246.htm http://www.path.org/our-work/vaccine-delivery.php http://www.universalgiving.org/donate/global_development_fund__wate/id6808.do http://www.qualitysurgeryindia.com/tag/best-physicians-in-india/
Health is a state of physical, mental and social well-being and not only the absence of disease or illness. http://mstinsonbc.wikispaces.com/1-2+-+Wellness+Triangle http://reviewhealthandfitness.com/
WHO’s Constitution sets out 3 priorities • Establishing legal agreements that set GLOBAL standards and norms
WHO’s Constitution sets out 3 priorities • Establishing legal agreements that set GLOBAL standards and norms • 2) Coordinating, leading, and directing decisions and projects on global health for countries within the UN
WHO’s Constitution sets out 3 priorities • Establishing legal agreements that set GLOBAL standards and norms • 2) Coordinating, leading, and directing decisions and projects on global health for countries within the UN • 3)Sharing and conducting reliable research and providing technical support to countries
People disagree about the relative priorities of these 3 roles http://www.realministry.org/priorities-priorities-part-2/
People disagree about the relative priorities of these 3 roles • WHO does carry out self-studies commissioned by the organization itself to determine what is best
In 1950, who had its first mass drive: tuberculosis http://www.redorbit.com/news/health/1112399010/tb-cases-globally-decline-for-the-first-time/
1990s: Dr. Fiona Godlee critiqued who’s main functions in the british medical journal http://www.scopeonline.co.uk/pages/meetings/volume_19_issue_4/senseaboutscience.shtml blacktrianglecampaign.org
As a result, who’s executive board held 6 meetings to review constitution http://www.globalhealth.gov/news-and-events/Press%20Release/OGA%20Greets%20African%20Diplomats.html http://deliver.jsi.com/dhome/newsdetail?p_item_id=26419376&p_token=B2816FBD195EBA82BD708400950A74FA&p_item_title=People%20that%20Deliver:%20Meeting%20Tomorrow%27s%20Health%20Challenges%20through%20Work%20Force%20Excellence%20in%20Supply%20Chain%20Management http://www.law.utoronto.ca/programs-centres/programs/international-human-rights-program/ihrp-summer-letters-2008/ihrp-letter-08 http://new.paho.org/hq/index.php?option=com_content&view=article&id=7263%3Ala-informacion-en-salud-en-camino-a-ser-un-bien-publico-regional-&catid=1443%3Anews-front-page-items&Itemid=1926&lang=en
WHO Partners with the Bill & Melinda Gates Foundation • And the Rockefeller Foundation http://gothamschools.org/2008/11/12/gates-foundation-will-steer-its-education-giving-in-a-new-direction-but-how-much-impact-will-the-billions-have/ http://en.wikipedia.org/wiki/Bill_%26_Melinda_Gates_Foundation http://www.ghananewsagency.org/details/Economics/Rockefeller-Foundation-to-foster-poverty-redu
There is tension within WHO regarding budget shifts and some criticize WHO for inefficiently using funds
View on Tobacco: the silent killer • WHO’s Tobacco Convention: world’s first tobacco control treaty http://tobacco.stanford.edu/tobacco_main/images.php?token2=fm_st007.php&token1=fm_img0162.php&theme_file=fm_mt001.php&theme_name=Doctors%20Smoking&subtheme_name=Dentists http://www.flickr.com/photos/41169113@N07/4859984418/
THROUGH POLIO INITIATIVE, WHO HAS REDUCED POLIO CASES BY 99% SINCE 1988 http://www.rotary.org/en/mediaandnews/news/pages/071031_news_polio.aspx
Some governments look up to WHO as health care role model today http://stopsmartmeters.org/2011/05/31/world-health-organization-declares-wireless-a-cancer-risk-smart-meter-mesh-network-must-be- http://getthisstrength.com/squats-looking-up-vs-looking-down
Potential questions to prepare for • Why was WHO created in the first place? • Who is in charge of WHO? President: Margaret Chan
Group of health scholars also examined who in 90s, concluding that who needed to update Global guidelines
World Health Organization
Published byEthel Manning Modified over 6 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
Action Plan for the Prevention of Avoidable Blindness and Visual Impairment Ivo Kocur World Health Organization
2 The Global Initiative for the Elimination of Avoidable Blindness
WHO The Global Initiative for the Elimination of Avoidable Blindness by 2020 Countries NGDOs IAPB
3 Global Magnitude of Blindness and Visual Impairment
314 million people visually impaired (VA 153 Million Uncorrected Refractive Errors (Avoidable) 161 Million Eye diseases (Avoidable or Not Avoidable) 49% 51% © WHO
5 Global Distribution of Blindness by Cause
Cataract 5% Glaucoma 18% Other 4% ARMD 50% Ch Bl 3% DR 17% CO Cataract 50 % Trachoma 4 % Glaucoma 12% Oncho 0.8 % Other 14 % ARMD 6% Ch Bl 4% DR 4% CO 5% More Developed Countries Less Developed Countries
6 World Health Assembly Resolutions
on prevention of blindness and visual impairment 2003: WHA 56.26 2006: WHA 59.25
7 VISION 2020 National Plans February 2009 Yes No No data
19 World Health Assembly 2009 Action Plan for the Prevention
of Avoidable Blindness and Visual Impairment
World Health Organization
Published byLauren Todd Modified over 7 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
19 April, 2017 Supplementary Training Modules on Good Manufacturing Practice Heating, Ventilation and Air- Conditioning (HVAC) Part 1 (a): Introduction and overview WHO Technical Report Series, No. 937, Annex 2
2 World Health Organization
HVAC 19 April, 2017 Objectives To understand: The need for HVAC systems (Part 1a) The role of HVAC in protection: Product Personnel Environment The role of HVAC in dust control (Part 1b) HVAC system design and its components (Part 2) Commissioning, qualification and maintenance (Part 3) 1. Introduction Heating, ventilation and air-conditioning (HVAC) play an important role in ensuring the manufacture of quality pharmaceutical products. A well designed HVAC system will also provide comfortable conditions for operators. These guidelines mainly focus on recommendations for systems for manufacturers of solid dosage forms. The guidelines also refer to other systems or components which are not relevant to solid dosage form manufacturing plants, but which may assist in providing a comparison between the requirements for solid dosage-form plants and other systems. HVAC system design infl uences architectural layouts with regard to items such as airlock positions, doorways and lobbies. The architectural components have an effect on room pressure differential cascades and cross-contamination control. The prevention of contamination and cross-contamination is an essential design consideration of the HVAC system. In view of these critical aspects, the design of the HVAC system should be considered at the concept design stage of a pharmaceutical manufacturing plant. Temperature, relative humidity and ventilation should be appropriate and should not adversely affect the quality of pharmaceutical products during their manufacture and storage, or the accurate functioning of equipment. This document aims to give guidance to pharmaceutical manufacturers and inspectors of pharmaceutical manufacturing facilities on the design, installation, qualifi cation and maintenance of the HVAC systems. These guidelines are intended to complement those provided in Good manufacturing practices for pharmaceutical products (1) and should be read in conjunction with the parent guide. The additional standards addressed by the present guidelines should therefore be considered supplementary to the general requirements set out in the parent guide. 2. Scope of document These guidelines focus primarily on the design and good manufacturing practices (GMP) requirements for HVAC systems for facilities for the manufacture of solid dosage forms. Most of the system design principles for facilities manufacturing solid dosage forms also apply to other facilities such as those manufacturing liquids, creams and ointments. These guidelines do not cover requirements for manufacturing sites for the production of sterile pharmaceutical products. These guidelines are intended as a basic guide for use by GMP inspectors. They are not intended to be prescriptive in specifying requirements and design parameters. There are many parameters affecting a clean area condition and it is, therefore, diffi cult to lay down the specifi c requirements for one particular parameter in isolation. Many manufacturers have their own engineering design and qualifi cation standards and requirements may vary from one manufacturer to the next. Design parameters should, therefore, be set realistically for each project, with a view to creating a cost-effective design, yet still complying with all regulatory standards and ensuring that product quality and safety are not compromised. The three primary aspects addressed in this manual are the roles that the HVAC system plays in product protection, personnel protection and environmental protection 1, 2
3 World Health Organization
HVAC 19 April, 2017 Introduction and Scope HVAC systems can have an impact on product quality It can provide comfortable conditions for operators The impact on premises and prevention of contamination and cross-contamination to be considered at the design stage Temperature, relative humidity control where appropriate Supplement to basic GMP text 1, 2
4 World Health Organization
19 April, 2017 HVAC Starting materials Personnel Procedures Validated processes Equipment Premises Environment Packing materials Factors contributing to quality products
6 World Health Organization
19 April, 2017 HVAC What is contamination? It is «the undesired introduction of impurities (chemical/ microbial/ foreign matter into or on to starting material or intermediate – during sampling, production, packaging or repackaging». Impurities could include products or substances other than the product manufactured, foreign products, particulate matter, micro- organisms, endotoxins (degraded microorganisms), etc. What are contaminants? Contaminants can originate from: Environment (particles, micro-organisms, dust containing other products). Equipment (residues of other products, oil, particles, rust, gaskets, metal) and can be brought into the product by air movements. Contaminants are in fact the presence of anything in the manufactured product which should not be there. Contaminants can be: Products or substances other than the product manufactured (e.g. products resulting from air pollution). Foreign products, such as metal parts from equipment, paint chips,etc. Particulate matter, especially dangerous in injectables. Micro-organisms – a particular problem for sterile products. Endotoxins: Even if killed by thermal treatment, micro-organisms are degraded to endotoxins and can cause damage. Glossary
8 World Health Organization
19 April, 2017 HVAC Cross-Contamination Contamination Contaminant from Environment Operators Equipment Cross Product
9 World Health Organization
19 April, 2017 HVAC Cross-contamination can be minimized by, e.g. Personnel procedures Adequate premises Use of closed production systems Adequate, validated cleaning procedures Appropriate levels of protection of product Correct air pressure cascade There are different ways to prevent or reduce the effect of cross-contamination. Personnel procedures: Clean clothing, and for clean rooms (C, B, A) non-linting clothing, to be washed in special laundries; Personal hygiene on entering a pharmaceutical area. Adequate premises: Minimisation of possibility of accumulation of dust; Premises with good ventilation and dedusting system. Closed production systems: Closed systems, in which product is transferred from one piece of equipment to another one, without being exposed to the atmosphere. Validated cleaning procedures: Manual cleaning procedures may not be reproducible. Level of Protection concept 2: A good hygiene, or Level of Protection concept, specifying requirements for environmental conditions; entry procedures for personnel and material is fundamental for keeping cross-contamination under control. Maintaining the correct air pressure differential between rooms helps prevent cross-contamination. The module on HVAC deals precisely with the last of these ways, namely a good air handling system.
10 World Health Organization
HVAC 19 April, 2017 The guideline further focuses on three concepts of the system: Product protection Contamination Cross-contamination Environmental conditions Personnel protection Prevent contact Comfort conditions Environment protection 2
11 World Health Organization
HVAC 19 April, 2017 Protection: Product and personnel Areas where materials and products are exposed, should be classified as «clean areas» Achievement of clean area classification depends on factors such as: Building finishes and structure Air filtration Air change rate Room pressure Temperature Relative humidity Material and personnel flow Outside environment Occupancy and type of product 4. Protection 4.1 Product and personnel 4.1.1 Areas for the manufacture of pharmaceuticals, where pharmaceutical starting materials and products, utensils and equipment are exposed to the environment, should be classifi ed as “clean areas”. 4.1.2 The achievement of a particular clean area classifi cation depends on a number of criteria that should be addressed at the design and qualifi cation stages. A suitable balance between the different criteria will be required in order to create an effi cient clean area. 4.1.3 Some of the basic criteria to be considered should include: • building fi nishes and structure • air fi ltration • air change rate or fl ushing rate • room pressure • location of air terminals and directional airfl ow • temperature • humidity • material fl ow • personnel fl ow • equipment movement • process being carried out • outside air conditions • occupancy • type of product.
12 World Health Organization
HVAC 19 April, 2017 Air filtration and air change rate should ensure attainment of classification Air change rate is dependent on factors, e.g. Level of protection required Quality and filtration of supply air Particulates generated Room configuration Containment effect Room heat load Room pressure Air change rate normally varies between 6 – 20 air changes per hour 4.1.4 Air fi ltration and air change rates should ensure that the defi ned clean area classifi cation is attained. 4.1.5 The air change rates should be determined by the manufacturer and designer, taking into account the various critical parameters. Primarily the air change rate should be set to a level that will achieve the required clean area classifi cation. 4.1.6 Air change rates normally vary between 6 and 20 air changes per hour and are normally determined by the following considerations: • level of protection required • the quality and fi ltration of the supply air • particulates generated by the manufacturing process • particulates generated by the operators • confi guration of the room and air supply and extract locations • suffi cient air to achieve containment effect • suffi cient air to cope with the room heat load • suffi cient air to maintain the required room pressure.
13 World Health Organization
HVAC 19 April, 2017 The classification should be achieved in the state as specified (1): «As built» Bare room, without equipment or personnel 4.1.7 In classifying the environment, the manufacturer should state whether this is achieved under “as-built” (Fig. 2), “at-rest” (Fig. 3) or “operational” (Fig. 4) conditions. 4.1.8 Room classifi cation tests in the “as-built” condition should be carried out on the bare room, in the absence of any equipment or personnel.
14 World Health Organization
HVAC 19 April, 2017 The classification should be achieved in the state as specified (2): «At rest» Equipment may be operating, but no operators present 4.1.9 Room classifi cation tests in the “at-rest” condition should be carried out with the equipment operating where relevant, but without any operators. Because of the amounts of dust usually generated in a solid dosage facility most clean area classify cations are rated for the “at-rest” condition. 4.1.9
15 World Health Organization
HVAC 19 April, 2017 The classification should be achieved in the state as specified (3): «In operation» Normal production process with equipment and personnel, Clean up time validated – normally in the order of 20 minutes Room classifi cation tests in the “operational” condition should be carried out during the normal production process with equipment operating, and the normal number of personnel present in the room. Generally a room that is tested for an “operational” condition should be able to be cleaned up to the “at-rest” clean area classifi cation after a short clean-up time. The clean-up time should be determined through validation and is generally of the order of 20 minutes. 4.1.10
16 World Health Organization
HVAC 19 April, 2017 Control of contaminants External contaminants removed through effective filtration Internal contaminants controlled through dilution and flushing, or displacement airflow Airborne particulates and level of filtration considered critical Materials and products should be protected from contamination and cross-contamination during all stages of manufacture (see also section 5.5 for cross-contamination control). Note: contaminants may result from inappropriate premises (e.g. poor design, layout or fi nishing), poor cleaning procedures, contaminants brought in by personnel, and a poor HVAC system. Airborne contaminants should be controlled through effective ventilation. External contaminants should be removed by effective fi ltration of the supply air (See Fig. 5 for an example of a shell-like building layout to enhance containment and protection from external contaminants.) Internal contaminants should be controlled by dilution and fl ushing of contaminants in the room, or by displacement airfl ow (See Figs 6 and 7 for examples of methods for the fl ushing of airborne contaminants.) Airborne particulates and the degree of fi ltration should be considered critical parameters with reference to the level of product protection required.
17 World Health Organization
19 April, 2017 HVAC Manufacturing Environment requirements Cleanroom Class A / B Cleanroom Class C Cleanrm. Class D The illustation shows that the manufacturing environmental requirements, as defined in the definition of the cleanroom zones, increase with the therapeutic risk. The Level of Protection classes are classified as a function of the product sensitivity to contamination (e.g. aseptically filled products are handled in a higher class than terminally sterilised products) and to the therapeutic risk (stricter environment for injectables, as injectables enter directly into the bloodstream without the additional protection given by the stomach and intestinal barriers ). In order to obtain a constant and well-defined quality level, it is necessary to have well-defined requirements for the cleanroom zones. Level of Protection classes are referred to as Class A, B, C, etc. in the EC countries, whereas other countries may refer to Class 100, 1000, etc or ISO Class 5, 6, 7, etc. These different classes will be discussed later in this module. Others Therapeutic risks
18 World Health Organization
HVAC 19 April, 2017 Level of protection and air cleanliness determined according to: Product to be manufactured Process to be used Product susceptibility to degradation The level of protection and air cleanliness for different areas should be determined according to the product being manufactured, the process being used and the product’s susceptibility to degradation (Table 1). 4.1.16
19 World Health Organization
19 April, 2017 HVAC Parameters influencing Levels of Protection Number of particles in the air, number of microorganisms in the air or on surfaces Number of air changes for each room Air velocity and airflow pattern Filters (type, position) Air pressure differentials between rooms Temperature, relative humidity The acceptable number of particles and the acceptable number of micro-organisms in the air is specified in the WHO guidelines, for the production of sterile products. It is also important to monitor surfaces for micro-organisms. The number of air changes are also described in the guidelines, but it should be noted that the WHO figures may differ from those of other guidelines such as EC and PIC/S. The air velocity is specified in the case of laminar flow installations (air flow pattern), should be in any case sufficient to achieve a proper flushing of the rooms and a short recovery (clean-up) time. Here too, there are differences between the WHO and other guidelines. The air flow patterns also influence the achievement of the hygiene class. Pressure differentials between rooms should be specified and monitored. In some cases, temperature and humidity can be critical for the product (e.g. effervescent tablets, hard gelatine capsules). In sterile areas, where people are heavily gowned, it is important to keep the temperature reasonably low, as people tend to perspire under a gown. Too low a humidity can bring static problems, with dust remaining “attached” to metal surfaces.
20 World Health Organization
19 April, 2017 HVAC Tools to help achieve the desired Level of Protection Air Handling System Production Room With Defined Requirements Supply Air Outlet Basically, an air handling system brings in air of a defined quality, in order to achieve an atmosphere of well-defined temperature, humidity and a defined limit of contamination, and evacuates the air after its passage through the concerned areas. Several parameters can be defined for cleanroom classes, which were mentioned in correlation with previous slides. Factors such as temperature and humidity must be also taken into account where necessary. It is imperative to define these parameters specifically for each cleanroom class and to remember that, within that given class, all defined parameters must be met. For each cleanroom class, these parameters are mainly controlled by the air handling system.
21 World Health Organization
19 April, 2017 HVAC Tools to help achieve the desired Level of Protection (2) Air-handling system can be the main tool for reaching required parameters May not be sufficient as such Need for additional measures such as appropriate gowning (type of clothing, proper changing rooms) validated sanitation adequate transfer procedures for materials and personnel Here are some examples of additional measures: Proper gowning, which must be adequately cleaned (lint-free clothing for clean-rooms C, B and A with special laundry and packaging under clean conditions). Good lockers for personnel, with separation between street and work clothing, and with adequate washing and disinfection facilities. Proper sanitation and hygiene practices (dust elimination, wet mopping, dedicated mops for different areas, rotating of disinfectants, etc.). Transfer procedures for material (decontamination measures, separate air locks for entering and outgoing goods, etc.). Proper premises.
22 World Health Organization
19 April, 2017 HVAC Tools to help achieve the desired Level of Protection (2) Cleanroom Class defined by Critical Parameters Air Handling System Additional Measures Whereas the air handling systems are the most important factor in creating the required environmental conditions for the Cleanroom classes, they alone cannot guarantee that the specifications corresponding to these classes will be met! Additional measures are therefore very important. We are going to discuss some of these measures.
23 World Health Organization
19 April, 2017 HVAC Examples of Levels of Protection Types of Clean room classes WHO, EC, PIC/S: A, B, C, D US FDA: Critical and controlled ISPE: Level 1, 2 or 3 ISO: Class 5, 6, 7 or 8 In order to have standardized requirements, regulatory bodies all over the world have defined some Cleanroom classes. The definition of various Cleanroom classes is mainly restricted to sterile manufacturing operations. WHO(*), EC and PIC/S and others mention classes A, B, C and D. The requirements for these classes differ slightly between WHO and EC. US FDA defines only 2 classes: critical and controlled. The ISPE refers to Level 1, 2 or 3 for non-sterile facilities and they refer to the cleanroom class for sterile facilities, ie. class 100, 1000 or ISO 5, 6 etc. There are no cleanroom classes defined by WHO or other regulatory bodies for the production of solids, liquids, creams, etc. It is nevertheless necessary to have one’s own cleanroom class descriptions for these production functions. The manufacturers must, therefore, create their own Level of Protection class definitions and their definitions must be such that the required product purity, as described in the pharmacopeias or in the registration documents, can be achieved at all times. (*) WHO Expert Committee on Specifications for Pharmaceutical Preparations. Thirty-Sixth Report. Geneva, World Health Organization, 2002 (WHO Technical Report Series, No. 902). Annex 6: Good manufacturing practices for sterile pharmaceutical products.
24 Comparing International Cleanroom Classifications
World Health Organization 19 April, 2017 HVAC Comparing International Cleanroom Classifications There are different norms for the number of particles in the air. The table (refer to handout ) shows that, for 0,5 micrometer particles, there are a number of names, those of the Federal Standard US 209 and the ISO guidelines being the most commonly in use at the moment. However, in the years to come, the ISO nomenclature will be generally adopted. The cleanroom classes for the pharmaceutical cleanrooms are highlighted in the table: We can see for instance that a Cleanroom class of type A corresponds to Non metric class 100 ( 100 particles / ft3 or particles / m3) Metric class 3,5 ( logarithmic calculation ) ISO class 5
25 World Health Organization
HVAC World Health Organization 19 April, 2017 Examples of levels of protection 4.1.16 Example of area Condition Level Area with normal housekeeping, e.g. warehouse General Level 1 Area where steps are taken to protect exposed material/product, e.g. dispensing Protected Level 2 Area with defined, controlled, monitored environmental conditions to prevent contamination and degradation Controlled Level 3 The level of protection and air cleanliness for different areas should be determined according to the product being manufactured, the process being used and the product’s susceptibility to degradation (Table 1).
26 World Health Organization
19 April, 2017 HVAC All operations within a pharmaceutical facilility should be correlated to well-defined clean room classes, and can be included in a hygiene concept. Example: etc. X Filling for aseptic process Filling for terminal sterilisation Depyrogenisation of containers Preparation of solutions for aseptic filling Preparation of solution for terminal sterilisation Washing of containers D C B A Cleanroom Class This slide describes a process for sterile products. Please note that this is an example only and protection requirements could be higher depending on the process and equipment used. For other pharmaceutical forms, similar tables have to be generated.
World Health Organization
Published byDevon Littles Modified over 7 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
Pharmaceutical Development 6 April, 2017 Training Workshop on Pharmaceutical Development with focus on Paediatric Formulations Tallinn 15-19th October 2007
2 Pharmaceutical Development
World Health Organization Pharmaceutical Development 6 April, 2017 Pharmaceutical Development of Finished Pharmaceutical Products (FPPs) Presenter: Susan Walters
3 The Australian view of the world
World Health Organization 6 April, 2017 The Australian view of the world We are here! This place isn’t too bad either!
4 What Australia gave to the world (1)
World Health Organization 6 April, 2017 What Australia gave to the world (1)
5 What Australia gave to the world (2)
World Health Organization 6 April, 2017 What Australia gave to the world (2)
6 Pharmaceutical Development of FPPs
World Health Organization Pharmaceutical Development of FPPs 6 April, 2017 Outline of presentation We will: Look at the development process as a whole & consider its objectives Review relevant guidelines Review sources of information Go through a worked example
7 Objectives of Pharmaceutical Development : What is the purpose?
World Health Organization 6 April, 2017 Objectives of Pharmaceutical Development : What is the purpose? From the perspective of a generic manufacturer, the objective is to develop a product that is: of appropriate quality & interchangeable with the innovator brand (so we can avoid expensive & time-consuming studies of safety & efficacy)
8 Objectives of Pharmaceutical Development : What is the purpose?
World Health Organization 6 April, 2017 Objectives of Pharmaceutical Development : What is the purpose? From the perspective of the manufacturer of a new dosage form &/or strength (eg a paediatric dosage form), the objective is to develop a product that is: Of appropriate quality, & Of appropriate dosage form & strength, & Either has been shown to be safe & effective for the claimed indications & patient population or has been shown to pharmacokinetically interchangeable with a brand that has been shown to be safe & effective for the claimed indications & patient population However safety & efficacy is outside the scope of this presentation so I will deal only with generics that contain the same API in the same dosage form & strength as the innovator
9 World Health Organization
6 April, 2017
10 World Health Organization
6 April, 2017 Product & process development (sorry don’t know the source of this diagram) CONTINUOUS IMPROVEMENT
11 Terminology – from ICH Q1A(R2) 2003 (stability)
World Health Organization Terminology – from ICH Q1A(R2) 2003 (stability) 6 April, 2017 Production batch: A batch of a drug substance or drug product manufactured at production scale by using production equipment in a production facility as specified Pilot scale batch: A batch of a drug substance or drug product manufactured by a procedure fully representative of and simulating that to be applied to a full production scale batch. For solid oral dosage forms, a pilot scale is generally, at a minimum, one-tenth that of a full production scale or 100,000 tablets or capsules, whichever is the larger. Laboratory scale batch [not an ICH definition] A batch smaller than pilot scale that is manufactured for development purposes Remember that scale-ups must be validated – batch characteristics may change during scale-up
12 Relevant non-WHO guidelines
World Health Organization Relevant non-WHO guidelines 6 April, 2017 ICH Q8 Pharmaceutical Development (2005) ICH Q9 Quality Risk Management (Nov 2005) ICH Q10 DRAFT Pharmaceutical Quality System (May 2007) Note for guidance on Process Validation CHMP/QWP/848/96 (EU 2001) An elderly guideline but informative & helpful
13 Relevant WHO guidelines
World Health Organization Relevant WHO guidelines 6 April, 2017 Pharmaceutical Development, Section 3.2 of Guideline on Submission of Documentation for Prequalification of Multi- source (Generic) Finished Pharmaceutical Products (FPPs) Used in the Treatment of HIV/AIDS, Malaria and Tuberculosis, WHO PQP (2005) Extension of the WHO List of Stable (not easily degradable ARV) APIs, Supplement 2 (Rev 1) to Guideline on Submission of Documentation for Prequalification of Multi-source (Generic) Finished Pharmaceutical Products (FPPs) Used in the Treatment of HIV/AIDS, Malaria and Tuberculosis, WHO PQP (2005) Supplementary guidelines on Good Manufacturing Practices: Validation, Annex 4 to WHO TRS 937 (2006)
15 Some relevant websites
World Health Organization Some relevant websites 6 April, 2017 WHO medicines program. WHO prequalification program. ICH website htm European guidelines for human medicines international Pharmaceutical Federation: Pharmaceutical Sciences section Dissolution methods for drug products
18 World Health Organization
What are the chemical & physicochemical properties of API(s) that we need to know, or are at least useful? 6 April, 2017 Solubility at various pH Acid or base? pKa & partition coefficient Stability under stress (eg oxygen, moisture, acid etc) Compatibility with common excipients
19 What literature should we look for?
World Health Organization What literature should we look for? 6 April, 2017 Look for…… A WHOPAR, if one is available for your product See Look for WHO Public Assessment Reports under Quick Links on the RH side of the page Innovator documentation. Can often be found on the innovator website. The prescribing information is especially useful & often includes a list of excipients. A drug approval package (DAP) via An EPAR (European Public Assessment Report) An official monograph in the Ph Int A monograph in Clarke’s Analysis of Drugs and Poisons, published by The Pharmaceutical Press (latest edition 2004). A monograph in The Merck Index, published by CambridgeSoft (latest edition 2001). Regulatory information See for example the WHO information line
29 What useful information did we find? – 7
World Health Organization 6 April, 2017 What useful information did we find? – 7 In vivo data provided by the innovator included the following : Nevirapine is readily absorbed (> 90 %) after oral administration in healthy volunteers & in adults with HIV-1 infection. A 3-way crossover study comparing the bioavailability of three production/commercial scale batches that had varying dissolution profiles showed that all three batches were bioequivalent with respect to systemic exposure (AUC). The statistically significantly different values for Cmax and tmax were considered not to be clinically relevant. In studies in which the suspension was administered directly using a syringe, it was demonstrated that the suspension & tablet formulations were comparably bioavailable with respect to extent of absorption. In a study in which the suspension was administered in a dosing cup without rinsing, the suspension intended for marketing was bioequivalent to the suspension used during clinical trials but was not bioequivalent to the marketed tablets. This was attributed to incomplete dosing of the two suspensions since there was about 13 % of the dose remaining in the cup.
30 What useful information did we find? – 8
World Health Organization 6 April, 2017 What useful information did we find? – 8 Based on adult experience, a comparable lead-in period of two weeks was suggested for paediatric population. A 4 mg/kg dose is proposed for all children regardless of age. Although no particular study has been performed to find the optimal lead-in dose, this dose was considered acceptable considering the enzyme induction to achieve initial antiretroviral activity. The following doses were approved: Patients from 2 months to 8 years, 4mg/kg once daily for 2 weeks followed by 7mg/kg twice daily Patients from 8 years to 16 years, 4 mg/kg once daily followed by 4mg/kg twice daily
31 World Health Organization
6 April, 2017 Benchmarking the innovator – 1 (these slides were taken from a presentation by János Pogány ) Obtain a sample for confirmation of characteristics Batch numbers Shelf life: 3 years and within 2 months of opening. Storage instructions: No special precautions for storage Container and closure system: as per EPAR QC analysis (hypothetical figures) Assay: 99.9% of labelled amount (LA) Methylhydroxy benzoate (HPLC): 0.18% w/v Propylhydroxy benzoate (HPLC): 0.02% w/v Total related substances: 0.03% Specific gravity (at 25oC): 1.150 Viscosity (at 25oC): 1,150 cPs pH: 5.80
33 Benchmarking the innovator – 3 Our tests show…..
World Health Organization 6 April, 2017 Benchmarking the innovator – 3 Our tests show….. % API dissolved (hypothetical figures) Time (minutes) 27 5 42 10 55 15 65 20 76 30 88 45 92 60 Dissolution profile (% labeled strength) Apparatus: USP II (paddle, 25rpm) Medium: 0.1N HCl Volume: 900ml See olution/dsp_SearchResults_Dissolutions.cfm downloaded on 13 March 2007
35 Pharmaceutical development protocol
World Health Organization 6 April, 2017 Pharmaceutical development protocol API experiments Particle size distribution Formulation experiments Screening laboratory batches with different proportions of excipients to match innovator dissolution Stress testing of the selected composition Compatibility with excipients Antimicrobial effectiveness test according to Ph Eur Packing materials Dimensions and tolerances of packing components Precision & accuracy of the dosing syringe
36 Product-specific API properties
World Health Organization Product-specific API properties 6 April, 2017 Ph Int specifications + limits on residual solvents from API manufacture Product-specific physical properties depend on crystallization and subsequent physical processing. Density and particle size distribution of nevirapine hemihydrate are critical quality attributes. Acceptance criteria are established by measurement of particle size of innovator’s API in suspension & through the similarity of dissolution profiles of innovator and generic products.
37 World Health Organization
6 April, 2017 Undertake stress testing of the API if not already available in existing documentation Stress type Conditions Assay (%) Control 25o C 99.8 36% HCl 80o C, 40 min. 72.0 5N NaOH 80o C, 2h 20’ 98.6 30% w/w H2O2 Heat 130o C, 49h 101.5 Light 500W/m2, 68h 101.7 Water 25o C, 92% RH, 91h 101.2
38 World Health Organization
Solubility of nevirapine hemihydrate at 37oC If not already available in existing documentation 6 April, 2017 Note: Nevirapine hemihydrate belongs to BCS2 (low solubility, high permeability) See Annex 8 to WHO TRS 937 (2006) Solubility data are also important for cleaning validation
39 Dissolution profiles of innovator & generic FPPs Hypothetical data
World Health Organization 6 April, 2017 Dissolution profiles of innovator & generic FPPs Hypothetical data M e a n % A P I d i s o l v ▀ innovator ▀ generic Similarity factor f2=73 Time (minutes)
40 Selected generic composition Hypothetical numbers
World Health Organization 6 April, 2017 Selected generic composition Hypothetical numbers Ingredients mg/5ml Nevirapine hemihydrate Excipients Carbomer 934P Methyl parahydroxybenzoate Propyl parahydroxybenzoate Sorbitol Sucrose (!) Polysorbate Sodium hydroxide q.s. Purified water to make ml
41 Proposed FPP specifications A hypothetical set of limits
World Health Organization 6 April, 2017 Proposed FPP specifications A hypothetical set of limits Description: including at least colour, texture, odour Identification (HPLC) Dissolution (UV): Q = 70% in 45 minutes pH = 5.0 – 6.1 Deliverable volume Average fill volume: NLT 240 ml Fill volume variation: Meets Ph Int requirements Related substances: NMT 0.1% of any one imp & NMT 0.3% total imps Preservative content (HPLC) Methylparaben: 98 to 102% of labeled strength Propylparaben: 98 to 102% of labeled strength Assay: 95.0 to 105.0% of labeled strength
42 World Health Organization
6 April, 2017 Compatibility with excipients May not need to do this if use only the same excipients as the innovator Nevirapine hemihydrate in solid state – illustrative example: heat
43 Development of manufacturing process
World Health Organization 6 April, 2017 Development of manufacturing process Select a standard process for oral aqueous suspensions, if possible using our existing method Manufacture a lab scale batch If necessary make adjustments & manufacture another lab scale batch When satisfied with the formulation, manufacture a pilot scale batch If necessary make adjustments & manufacture another pilot scale batch Recollect that a pilot batch is manufactured by a procedure fully representative of and simulating that to be applied to a full production scale batch Manufacture primary* batches in the proposed container & closure systems for: Bioequivalence & dissolution studies Regulatory stability studies Iincluding an in-use stability study & a stress study under freeze-thaw conditions Validation of bioequivalence, dissolution & stability batches *Primary as defined in WHO/ICH guidelines
44 Development of manufacturing process
World Health Organization 6 April, 2017 Development of manufacturing process Note that the progress from pre-formulation to formulation to pilot manufacture to production scale manufacture should be described in the PQP dossier & shown to be logical, reasoned & continuous
45 World Health Organization
6 April, 2017 Scale up activities Test a large number of samples from pilot scale batches to establish provisional acceptance limits for the control of critical process parameters (prospective validation, in-process control limits) in order to define the design space* & a control strategy that encompasses batch scale, equipment, packaging, as well as final product stability. The process will be well understood when: all critical sources of variability have been identified & explained variability is managed by the process product quality attributes can be accurately & reliably predicted A validation protocol is written * See ICH Q8, Q9 & draft Q10 for further explanation
46 Dissolution & bioequivalence testing
World Health Organization 6 April, 2017 Dissolution & bioequivalence testing Innovator FPP Generic FPP Conduct a dissolution test on at least 3 batches Select a production batch, or one NLT 1/10th of final size Select a batch showing intermediate dissolution Reference product Test product Dissolution profile Bioequivalence study
47 Pharmaceutical Development Summary and conclusion
World Health Organization Pharmaceutical Development Summary and conclusion 6 April, 2017 The probability of producing a product that is: Of high quality Stable Consistent from batch to batch, & Bioequivalent to the innovator can be significantly improved by employing a planned & systematic approach to product development, & using all the information that has been published
World Health Organization
Published byDominick Austin Modified over 7 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
15 April, 2017 Essential Newborn Care Dr P. Mongi African Regional Meeting on Interventions for Impact for Essential Obstetric and Newborn Care: February 2011, Addis Ababa, Ethiopia
2 Presentation outline Some Facts and Figures on Newborn health
Essential Newborn Care Definition of Essential Newborn Care Key components of Essential Newborn Care WHO Essential Newborn Care Course- ENCC & Resuscitation
3 Progress towards MDG 4 Total number of deaths of children Neonatal mortality represents about 40% (3·575 million), most in the first week
7 In Africa 1·2 Neonatal Deaths: Why?
Lower contribution to overall child mortality Prematurity and birth asphyxia contribute equally Neonatal deaths likely to increase as child deaths fall CHERG Lancet 2010; 375: 1969–87
8 Causes of Newborn deaths in SS-Africa
Newborn deaths constitute 29% of under five mortality The main causes for almost 90% of Newborn deaths are: Infections- 32% Prematurity and Low birth weight- 29% Birth Asphyxia and birth trauma- 27% ALL are preventable and treatable MDG- 4 can only be achieved if neonatal deaths are addressed and this necessitates both maternal and child health interventions
10 World Health Organization
Coverage of key Maternal and Child Survival Interventions, in WHO/AFRO 2008 15 April, 2017 The hours and days of highest risk have the lowest coverage of care Source: WHO World Health Statistics 2010, UNICEF State of the World’s Children, 2010 10
12 Essential Newborn Care-Components
World Health Organization 15 April, 2017 Cleanliness Thermal protection Early and exclusive breastfeeding Initiation of breathing, resuscitation Eye care Immunization Management of newborn illness Care of the preterm and/or low birth weight newborn The WHO definition stemms from a WHO Working Group Meeting in 1994 in Trieste, Italy. ENC was defined as consisting of eight components: Cleanliness: clean delivery and clean cord care for the prevention of newborn infections Thermal protection: prevention and/or management of neonatal hypothermia and hyperthermia Early and exclusive breastfeeding: Breasfeeding should be started within an hour of birth. Feeding should be as frequent as the baby demands, without prelacteal feeds or other fluids and food. Knowledge about importance of breastfeedin should be disseminated among families and communities as well as health workers and managers (10 steps) Initiation of breathing, resuscitation: «Helping Babies Breathe» … These elements remain, even if some of these elements have slightly changed in content, sine. The concept of ENC was further divided into Basic Care and Special Care. Basic Care includes interventions for all infants to meet their physiological needs. Special Care is required for a small group of newborns because of diseases acquired before, during or after birth and/or because they are born too soon or too small. This was the basis for developing the essential practice guide of the Integrated Management of Pregnancy and Childbirth (IMPAC) series as well as the guide for doctors, nurses and midwives Managing Newborn Problems.
13 Integrated Management of Pregnancy and Childbirth (IMPAC)
World Health Organization Integrated Management of Pregnancy and Childbirth (IMPAC) 15 April, 2017 Clinical guidelines Programme guides Education modules Advocacy material Tools for monitoring and evaluation
14 Essential Newborn Care Course
New layout French version in print
15 The 5 modules in the ENCC 1. Care of the baby at the time of birth
Introduction to the PCPNC guide Universal precaution Care of the baby at birth Keeping baby warm 2. Examination of the newborn baby Breasting a newborn baby- ensuring a good start Communication skills Examination of the newborn 3. Care of the newborn baby until discharge Resuscitation of the newborn baby Routine care of the newborn baby 4. Special situations Overcoming difficulties in breastfeeding The small baby Alternative methods of feeding a baby 5. Optional module Giving an injection Kangaroo mother care
16 The WHO Essential Newborn Care Course
World Health Organization The WHO Essential Newborn Care Course 15 April, 2017 Five days course on essential and emergency newborn care Any health facility dealing with mothers and newborns For doctors, midwives and nurses
17 ENCC: State of Implementation
World Health Organization ENCC: State of Implementation 15 April, 2017 Mali Kenya No WHO AFRO Trained trainers available (8 countries) Al least one core national ENC training conducted (7 countries) 4 districts Training started in periphery (3 countries) 7 or more district/provincial courses (2 countries) By 2010 ENCC training has been introduced in 40 countries globally AFRO-20 EMRO SEARO WPRO Yoy will receive an updated slide
18 Effective Perinatal Care Package (EPCP)
Total 15 countries
19 Asphyxia &Resuscitation of the newborn baby
Asphyxia is failure to breath within one minute after delivery. Good labour could prevent most birth asphyxia This is treatable if births are attended by health worker skilled in neonatal resuscitation One in 20 (5%) babies need help with breathing- resuscitation Resuscitation must be anticipated at each delivery Resuscitation skills are essential to the survival of babies
20 Training Newborn Resuscitation in the Context of Essential Newborn Care-1
Newborn resuscitation is a critical component of ENC, but one out of the eight components The WHO ENCC training package contains a module on basic newborn resuscitation based on the 1998 guidelines currently updated Countries may chose to replace that module by Helping Babies Breath (HBB) The introduction of ENCC should trigger the review of the national neonatal resuscitation guidelines including by level of care
World Health Organization (WHO)
World Health Organization (WHO). Jason A. Gonzalez BIOL 402 April 13, 2010. WHO is the global health branch of the UN. Their agenda is ambitious. The WHO’s goal is world peace through global health and security. The WHO’s major task is to combat disease, especially key infectious diseases.
World Health Organization (WHO)
Presentation Transcript
World Health Organization (WHO) Jason A. Gonzalez BIOL 402 April 13, 2010
The WHO agenda has six points: • Two health objectives • Two strategic needs • Two operational approaches
WHO promotes development of 3rd world and impoverished nations and increases access to health care • Equity
Epidemics Revised International Health Regulations (June 2007) WHO fosters global health security and monitors potential pandemic situations.
WHO funds and emphasizes research to more efficiently and appropriately address global health problems. • Authoritative health information
The WHO partners and collaborates with existing organizations • International organizations • Civil society • Private sector
They continuously seeks to improve standards and performance of global health policy and administration • Ongoing reforms
General success is measured in the state of women’s health and health in Africa Is it successful?
The WHO communicates their projects, funding, and research knowledge through their publications • International Classification of Diseases (ICD) • World Health Report (annual) • Bulletin of the World Health Organization (monthly) • List of Essential Medicines • Global plan of action on worker’s health • Health Sciences Online
The WHO is not an original. • 1907-1946 (1950): The Office International d’Hygiene Publique (OIHP)
April 7, 1948: WHO • Happy World Health Day!
Smallpox (1980) Cholera Typhoid Malaria SARS HIV The WHO will kill again.
The Global Malaria Program (GMP) provides technical assistance to countries to combat malaria on a global scale.
Home Management of Malaria (HMM) • Access to anti-malarial medications • Training for community-based service providers • Education and communication • Supervision
The WHO faces some criticism for overreaching their scope with regulations. • Tobacco regulation • Sugar
The WHO has been accused of neglecting donor nations in need. • Selling viruses to independent labs to make vaccines (and patents!)
The WHO’s national health rankings may be misleading. • Rankings • Overall Attainment • US – 37 • Overall Performance • US – 14 • Financial Fairness Factor • Percentage of income spent on health care • Wider dispersion lowers score #37!
The WHO has big plans for the future • Millennium Development Goals • 8 goals to achieve by 2015
World Health Organization
Published byMagdalen George Modified over 7 years ago
Similar presentations
Presentation on theme: «World Health Organization»— Presentation transcript:
1 World Health Organization
15 April, 2017 Supplementary Training Modules on Good Manufacturing Practice Good Practices for Quality Control Laboratories Part 2: Materials, equipment, instruments and other devices
2 World Health Organization
Quality Control 15 April, 2017 Reagents Reagents, chemicals, solvents and materials used in tests and assays – of appropriate quality – with COA and MSDS From reputable, approved suppliers Preparation of reagents: SOPs and as recommended in pharmacopoeia Clear responsibility in job descriptions Records for the preparation, and standardization of volumetric solutions 10. Reagents 10.1 All reagents and chemicals, including solvents and materials used in tests and assays, should be of appropriate quality. 10.2 Reagents should be purchased from reputable, approved suppliers and should be accompanied by the certificate of analysis, and the material safety data sheet, if required. 10.3 In the preparation of reagent solutions in the laboratory: (a) responsibility for this task should be clearly specified in the job description of the person assigned to carry it out; and (b) prescribed procedures should be used which are in accordance with published pharmacopoeial or other standards where available. Records should be kept of the preparation and standardization of volumetric solutions. 10.1 – 10.3
3 World Health Organization
Quality Control 15 April, 2017 Reagents clearly labelled: the contents, the manufacturer, the date received and opened, concentration, storage conditions, expiry or re-test date When prepared in the laboratory, also the name, date of preparation, initials of person, expiry date, concentration Volumetric solutions: the name, molarity or concentration, date of preparation, the date of standardization and factor, and identify the responsible technician Whenever possible, transportation in original containers When subdivided – into clean, fully labelled containers 10.4 The labels of all reagents should clearly specify: (a) content; (b) manufacturer; (c) date received and date of opening of the container; (d) concentration, if applicable; (e) storage conditions; and (f) expiry date or retest date, as justified. 10.5 The labels of reagent solutions prepared in the laboratory should clearly specify: (a) name; (b) date of preparation and initials of technician or analyst; (c) expiry date or retest date, as justified; and (d) concentration, if applicable. 10.6 The labels for volumetric solutions prepared in the laboratory should clearly specify: (b) molarity (or concentration); (c) date of preparation and initials of technician/analyst; (d) date of standardization and initials of technician/analyst; and (e) standardization factor. Note: The laboratory should ensure that the volumetric solution is suitable for use at the time of use. 10.7 In the transportation and subdivision of reagents: (a) whenever possible they should be transported in the original containers; and (b) when subdivision is necessary, clean containers should be used and appropriately labelled.
4 World Health Organization
Quality Control 15 April, 2017 Visual inspection Ensure seals are intact (receiving, distribution for use) Suggested to record this on the label (e.g. date, name and initial) If tampered with, rejected, unless identity and purity can be confirmed Water Considered as a reagent – use grade as in pharmacopoeia Precautions to avoid contamination during: supply, storage and distribution Meet specification Water 10.10 Water should be considered as a reagent. The appropriate grade for a specific test should be used as described in the pharmacopoeias or in an approved test when available. 10.11 Precautions should be taken to avoid contamination during its supply, storage and distribution. 10.12 The quality of the water should be verified regularly to ensure that the various grades of water meet the appropriate specifications.
5 World Health Organization
Quality Control 15 April, 2017 Storage of reagents Appropriate storage conditions Store also clean bottles, vials, spoons, funnels, and labels required for dispensing reagents from larger to smaller containers Training in safe handling of chemicals and reagents Store keeper responsibilities: Appropriate store facility, inventory, expiry dates Storage 10.13 Stocks of reagents should be maintained in a store under the appropriate storage conditions (ambient temperature, under refrigeration or frozen). The store should contain a supply of clean bottles, vials, spoons, funnels and labels, as required, for dispensing reagents from larger to smaller containers. Special equipment may be needed for the transfer of larger volumes of corrosive liquids. 10.14 The person in charge of the store is responsible for looking after the storage facilities and their inventory and for noting the expiry date of chemicals and reagents. Training may be needed in handling chemicals safely and with the necessary care. 10.13 – 10.14
6 World Health Organization
Quality Control 15 April, 2017 Reference substances and reference materials A person nominated responsible Use pharmacopoeia reference substances whenever possible Used for testing, calibration, qualification of equipment, instruments or other devices Registration and labelling Identification number assigned a new identification number to each new batch number marked on each vial and quoted on the analytical worksheet at every use (batch number) 11. Reference substances and reference materials 11.1 Reference substances (primary reference substances or secondary reference substances (8)) are used for the testing of a sample. Note: Pharmacopoeial reference substances should be employed when available and appropriate for the analysis. When a pharmacopoeia reference substance has not been established then the manufacturer should use its own reference substance. 11.2 Reference materials may be necessary for the calibration and/or qualification of equipment, instruments or other devices. Registration and labelling 11.3 An identification number should be assigned to all reference substances, except for pharmacopoeial reference substances. 11.4 A new identification number should be assigned to each new batch. 11.5 This number should be marked on each vial of the reference substance. 11.6 The identification number should be quoted on the analytical worksheet every time the reference substance is used (see Part three, section 15.5). In the case of pharmacopoeial reference substances the batch number and/or the batch validity statement should be attached to the worksheet. 11.1. – 11.6, 11.8.
7 World Health Organization
Quality Control 15 April, 2017 Maintain a register for reference substances and reference materials. Record e.g.: (1) identification number of the material precise description of the material source date of receipt batch designation or other identification code intended use of the material (e.g. as an infrared reference material, as an impurity reference material for thin-layer chromatography, etc.) location of storage in the laboratory, and any special storage conditions 11.7 The register for all reference substances and reference materials should be maintained and contain the following information: (a) the identification number of the substance or material; (b) a precise description of the substance or material; (c) the source; (d) the date of receipt; (e) the batch designation or other identification code; (f) the intended use of the substance or material (e.g. as an infrared reference substance or as an impurity reference substance for thin-layer chromatography); (g) the location of storage in the laboratory, and any special storage conditions; (h) any further necessary information (e.g. the results of visual inspections); (i) expiry date or retest date; (j) certificate (batch validity statement) of a pharmacopoeial reference substance and a certified reference material which indicates its use, the assigned content, if applicable, and its status (validity); and (k) in the case of secondary reference substances prepared and supplied by the manufacturer, the certificate of analysis. 11.7
9 World Health Organization
Quality Control 15 April, 2017 Reference substances prepared in the laboratory: Keep a file with information (properties and safety data) Results of all tests and verifications Expiry date or retest date Signed by the responsible analyst 11.9 If a national pharmaceutical quality control laboratory is required to establish reference substances for use by other institutions, a separate reference substances unit should be established. 11.10 In addition a file should be kept in which all information on the properties of each reference substance is entered including the safety data sheets. 11.11 For reference substances prepared in the laboratory, the file should include the results of all tests and verifications used to establish the reference substances and expiry date or retest date; these should be signed by the responsible analyst. 11.9. –
11 World Health Organization
Quality Control 15 April, 2017 Calibration, validation and verification of equipment, instruments and other devices Unique identification (number) for equipment, instruments, devices used for testing, verification and/or calibration Also labels indicating status of calibration and due date DQ, IQ, OQ, PQ as necessary Performance verified at appropriate intervals according to a plan Plan for regular calibration implemented 12.1 Each item of equipment, instrument or other device used for testing, verification and/or calibration should, when practicable, be uniquely identified. 12.2 All equipment, instruments and other devices (e.g. volumetric glassware and automatic dispensers) requiring calibration should be labelled, coded or otherwise identified to indicate the status of calibration and the date when recalibration is due. 12.3 Laboratory equipment should undergo design qualification, installation qualification, operation qualification and performance qualification (for definitions of these terms see the Glossary) (11). Depending on the function and operation of the instrument, the design qualification of a commercially available standard instrument may be omitted as the installation qualification, operational qualification and performance qualification may be considered to be a sufficient indicator of its suitable design. 12.4 As applicable, the performance of equipment should be verified at appropriate intervals according to a plan established by the laboratory.
12 World Health Organization
Quality Control 15 April, 2017 Examples for calibration: SOPs should be in place for each instrument pH meters to be verified with standard certified buffer solution before use Balances calibrated annually, verified daily, and regularly checked with test weights What do you think should be appropriate intervals for the calibration and verification of: HPLCs, GCs, FTIRs? 12.6 Specific procedures should be established for each type of measuring equipment, taking into account the type of equipment, the extent of use and supplier’s recommendations. For example: — pH meters are verified with standard certified buffer solutions before use; — balances are to be checked daily using internal calibration and regularly using suitable test weights, and requalification should be performed annually using certified reference weights. Discuss parameters and intervals for calibration and verification of other instruments Note: For further guidance on calibration, verification of performance and qualification of equipment refer to: • Procedures for verifying and calibrating refractometers, thermometers used in determinations of melting temperatures and potentiometers for pH determinations and methods for verifying the reliability of scales for ultraviolet and infrared spectrophotometers and spectrofluorometers in The International Pharmacopoeia (19); • Specific guidelines for qualification of equipment elaborated by the European Network of Official Medicines Control Laboratories (OMCL) (20); and • General chapter of the US Pharmacopeia on Analytical instrument qualification (21). 12.6.
13 World Health Organization
Quality Control World Health Organization 15 April, 2017 In the following slides, we will look at some instruments you may find in the quality control laboratory Discuss which parameters may be considered during calibration and verification Discuss appropriate intervals for calibration and verification Discuss some specific aspects to be observed when using the apparatus In the following slides, we will look at some instruments you may find in the quality control laboratory Discuss which parameters may be considered during calibration and verification Discuss appropriate intervals for calibration and verification Discuss some specific aspects to be observed when using the apparatus The trainer should know the instruments and parameters that are to be included in calibration and verification. See references in relevant pharmacopoeia and discuss these with the trainees
14 World Health Organization
Quality Control World Health Organization 15 April, 2017 Dissolution apparatus Dissolution: Setting up the apparatus The head plate, which holds the shafts, and the base plate, which holds the vessels, must be leveled to maintain co-planarity. It should also be checked with a spirit level in the two horizontal planes. It is essential that the basket shafts be vertical, that the vessels be resting on a horizontal plane and that the vessels be centered properly. After centering, each basket must be individually adjusted to a position mm from the bottom of the vessel. The laboratory should ensure that all portions of the apparatus that will come into contact with the dissolution medium are thoroughly cleaned prior to use. The dissolution medium should be warmed to oC and de-aerated immediately prior to transfer. If graduated cylinders are used to dispense the dissolution medium they should be calibrated prior to use. Use of volumetric glassware is preferred. Calibration of the apparatus: check the frequency of calibration, the use of chemical calibration and calibration tablets. Check the preparation of the dissolution media. There should be traceability to a record sheet. Testing errors will all make the product appear to perform better than it is. They include factors such as: Influence of temperature: The thermal distribution profile of the water bath should be checked. Note that turbidity in the water could indicate growth of bacteria Rotational speed: Check calibration with a tachometer. Sources of vibration: Vibration causes turbulence. Sometimes a shock absorbing rubber mat is used under the apparatus and the drive unit may have good vibration-limiting shock absorbers. Shaft wobble will mean turbulence. Shaft Perpendicularity: Check with a spirit level and a builder’s square. Tension on the chain or belt. Looseness will mean speeding up and slowing down leading to turbulence. Bubbles: Can create turbulence. Acid on unprotected steel (even stainless steel) can lead to bubbles of hydrogen gas. Shafts must be centered with respect to the vessels.
15 World Health Organization
Quality Control World Health Organization 15 April, 2017 Friability tester Friability testing may be an in-process control test, a finished product specification test or both. Limits may be in the marketing authorisation or pharmacopoeial limits may be applied.
16 World Health Organization
Quality Control 15 April, 2017 Disintegration tester
17 World Health Organization
Quality Control World Health Organization 15 April, 2017 HPLC: Column performance: A column performance check should be designed to measure chromatographic efficiency, capacity and symmetry. Check at intervals and also whenever significant changes in peak shape, resolution or retention time are observed during an analysis. System suitability test: The performance of an HPLC system should be assured before starting an assay using a method employing an external or internal standard. There should be at least 5 injections of standard solution. These injections may be distributed throughout the analytical run. The relative standard deviation for peak areas and retention times should be less than 2% and 1% respectively. The resolution, R, between reference and internal standard peaks should be specified and calculated from a suitable equation. Systems record: A log book should be assigned to each component and/or HPLC system. The first page of the log book should list each component of the system with an individual identification number. The following information should be recorded in a manner which is readily retrievable – date, analyst, mobile phase used, duration of use, problem reports and corrective action (repairs) taken to rectify the problem, details of any replacement of existing components with spares including changeover of columns, results of column performance and system suitability checks, date and results of calibration checks, name and unique identification number of sample analyzed. Column Care: There should be adequate SOPs and records on column care, which should include washing storage and transport. Dropping columns or vibration will result in channels and voids. Use of dedicated columns is also recommended.
18 World Health Organization
Quality Control World Health Organization 15 April, 2017 Analytical balance Analytical balances Things to consider: Are they kept on or switched off when not in use; Internal calibration versus external calibration Calibration versus verification Accuracy, precision, range, eccentricity Standard weights use Frequency Number of decimals Cleanliness, level, vibration and shock as well as temperature and air flow patterns that may influence performance
19 World Health Organization
Quality Control World Health Organization 15 April, 2017 Spectrophotometer Ultraviolet & visible spectrophotometers: There should be a specification for the Cells, which should be a matched pair. Cleanliness is critical for this glassware. The calibration and performance of the Spectrophotometer should be checked periodically, usually monthly until stability is demonstrated: frequency wavelength Accuracy photometric Accuracy line flatness stray light requirement resolution power Spectral wavelength standards can include holmium preparations (caution toxicity) and didymium filters. The spectral lines of the deuterium lamp also serve as reference points. FTIR, IR spectrophotometers: Calibration: Performance tests should be carried out regularly to ensure that the instrument is in working order, about on a monthly basis. If this routine monthly data shows the performance of the instrument has been reliable and stable over a 12 months period, then the interval between calibrations may be extended. After repair, stability of performance should again be demonstrated. Line flatness: Check the line (100 per cent transmittance line) in accordance with the instructions of the manufacturer. The Io line should not deviate from a horizontal line at any point by more than the amount stated in the manufacturer’s specifications. Wave number calibration and general instrument performance check: A polystyrene film of appropriate thickness (commonly 50um) should be used. Run a spectrum under operating conditions which match those used for running sample spectra. The positions of the absorption peaks should not differ from the values given by more than 10 cm-1 in the region cm-1, or by more than 3 cm-1 in the region cm-1. Performance check and spectral resolution should be checked by measuring peak heights of closely aligned peaks on the polystyrene film. The wave number of the peaks is given in the various pharmacopoeia.
20 World Health Organization
Quality Control World Health Organization 15 April, 2017 pH Meter Because it is so “simple” it may be overlooked in terms of an SOP on how to use it. If the pH meter does not have temperature compensation, ensure that the SOP states the temperature of the test solution. Usually, verification before use with different buffer use Verify how buffer solutions are prepared, stored, shelf life, traceability
21 Inspecting the QC laboratory
During your inspection of the QC laboratory you come across analytical equipment you are not familiar with. What questions could you ask? Inspectors will come across analytical equipment and techniques with which they are not familiar. This is a good learning opportunity. Some questions would be: 1. Please show me the SOP for this analysis (The SOP should explain the basis of the analysis) 2. Ask to see the calibration SOP (This will provide further technical details) 3. Ask the analyst “What are the typical analytical problems you see? For example, is sample preparation a problem? What are the key variables with this technique?
22 World Health Organization
Quality Control 15 April, 2017 Authorized personnel to operate equipment SOPs readily available for use, maintenance, verification and calibration of equipment, instruments and devices Manuals kept The results of calibration and verification recorded SOPs for the safe handling, transport and storage of measuring equipment SOP for maintenance e.g. regular servicing followed by verification of performance. 12.7 Only authorized personnel should operate equipment, instruments and devices. Up-to-date SOPs on the use, maintenance, verification, qualification and calibration of equipment, instruments and devices (including any relevant manuals provided by the manufacturer) should be readily available for use by the appropriate laboratory personnel together with a schedule of the dates on which verification and/or calibration is due. 12.9 Procedures should include instructions for the safe handling, transport and storage of measuring equipment. On reinstallation, requalification of the equipment is required to ensure that it functions properly. 12.10 Maintenance procedures should be established, e.g. regular servicing should be performed by a team of maintenance specialists, whether internal or external, followed by verification of performance. 12.7. –
23 World Health Organization
Quality Control 15 April, 2017 Records kept of each item of equipment/ instrument/ device: Identity of the equipment, instrument or other device Manufacturer’s name, model, serial number or other unique identification Qualification, verification and/or calibration required Current location Equipment manufacturer’s instructions 12.8 Records should be kept of each item of equipment, instrument or other device used to perform testing, verification and/or calibration. The records should include at least the following: (a) the identity of the equipment, instrument or other device; (b) the manufacturer’s name and the equipment model, serial number or other unique identification; (c) the qualification, verification and/or calibration required; (d) the current location, where appropriate; (e) the equipment manufacturer’s instructions, if available, or an indication of their location; (f) the dates, results and copies of reports, verifications and certificates of all calibrations, adjustments, acceptance criteria and the due date of the next qualification, verification and/or calibration; (g) the maintenance carried out to date and the maintenance plan; and (h) a history of any damage, malfunction, modification or repair. It is also recommended that records should be kept and additional observations made of the time for which the equipment, instruments or devices were used. 12.8.
24 World Health Organization
Quality Control 15 April, 2017 Records kept of each item of equipment/instrument/device: Dates, results and copies of reports, verifications and certificates of all calibrations, adjustments, acceptance criteria and the due date of the next qualification, verification and/or calibration Maintenance carried out, and the maintenance plan History of any damage, malfunction, modification or repair Use and “remarks or observations” made at the time the equipment, instruments or devices were used 12.8 Records should be kept of each item of equipment, instrument or other device used to perform testing, verification and/or calibration. The records should include at least the following: (a) the identity of the equipment, instrument or other device; (b) the manufacturer’s name and the equipment model, serial number or other unique identification; (c) the qualification, verification and/or calibration required; (d) the current location, where appropriate; (e) the equipment manufacturer’s instructions, if available, or an indication of their location; (f) the dates, results and copies of reports, verifications and certificates of all calibrations, adjustments, acceptance criteria and the due date of the next qualification, verification and/or calibration; (g) the maintenance carried out to date and the maintenance plan; and (h) a history of any damage, malfunction, modification or repair. It is also recommended that records should be kept and additional observations made of the time for which the equipment, instruments or devices were used. 12.8.
26 World Health Organization
Quality Control 15 April, 2017 Traceability Result of analysis should be traceable (reported data, raw data, instruments, materials and reagents used) Includes traceability to a primary reference substance Also needed in case of OOS for investigations Calibrations or qualification of instruments should be traceable to certified reference materials and to SI units 13. Traceability 13.1 The result of an analysis should be traceable, when appropriate, ultimately to a primary reference substance. 13.2 All calibrations or qualification of instruments should be traceable to certified reference materials and to SI units (metrological traceability). 13.1. – 13.2.
Источники:
- http://www.myshared.ru/slide/1363027/
- http://slideplayer.com/slide/4337103/
- http://slideplayer.com/slide/5886619/
- http://ppt4web.ru/medicina/vsemirnaja-organizacija-zdravookhranenija.html
- http://slideplayer.com/slide/1423548/
- http://slideplayer.com/slide/7223444/
- http://thepresentation.ru/uncategorized/world-health-organization-who
- http://slideplayer.com/slide/5741292/
- http://www.slideserve.com/tymon/world-health-organization
- http://slideplayer.com/slide/6257483/
- http://www.slideserve.com/jam/world-health-organization
- http://www.myshared.ru/slide/1318321/
- http://www.slideserve.com/hwilliamson/world-health-organization-powerpoint-ppt-presentation
- http://www.myshared.ru/slide/824608/
- http://www.slideserve.com/shaun/world-health-organization
- http://slideplayer.com/slide/8081392/
- http://slideplayer.com/slide/5902408/
- http://slideplayer.com/slide/2582589/
- http://slideplayer.com/slide/4549439/
- http://www.slideserve.com/chesmu/world-health-organization-who
- http://slideplayer.com/slide/4587820/